Abstract
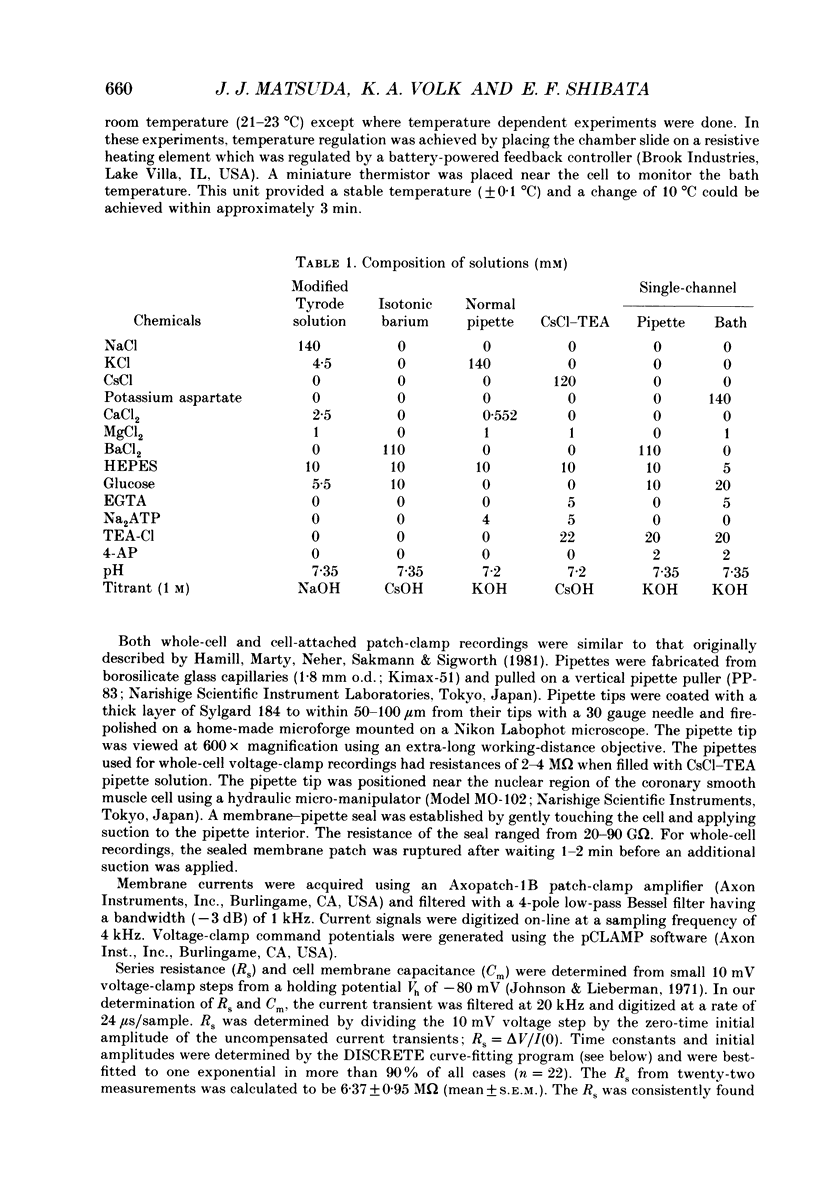
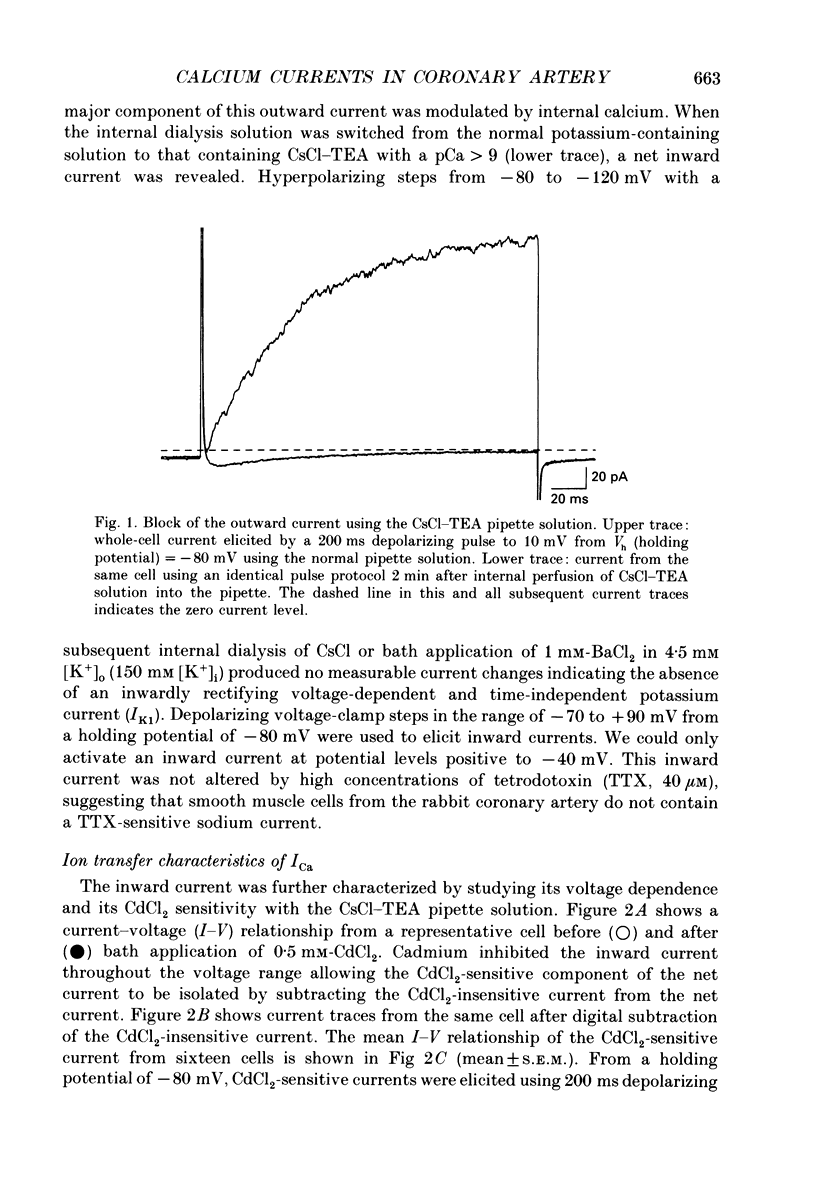
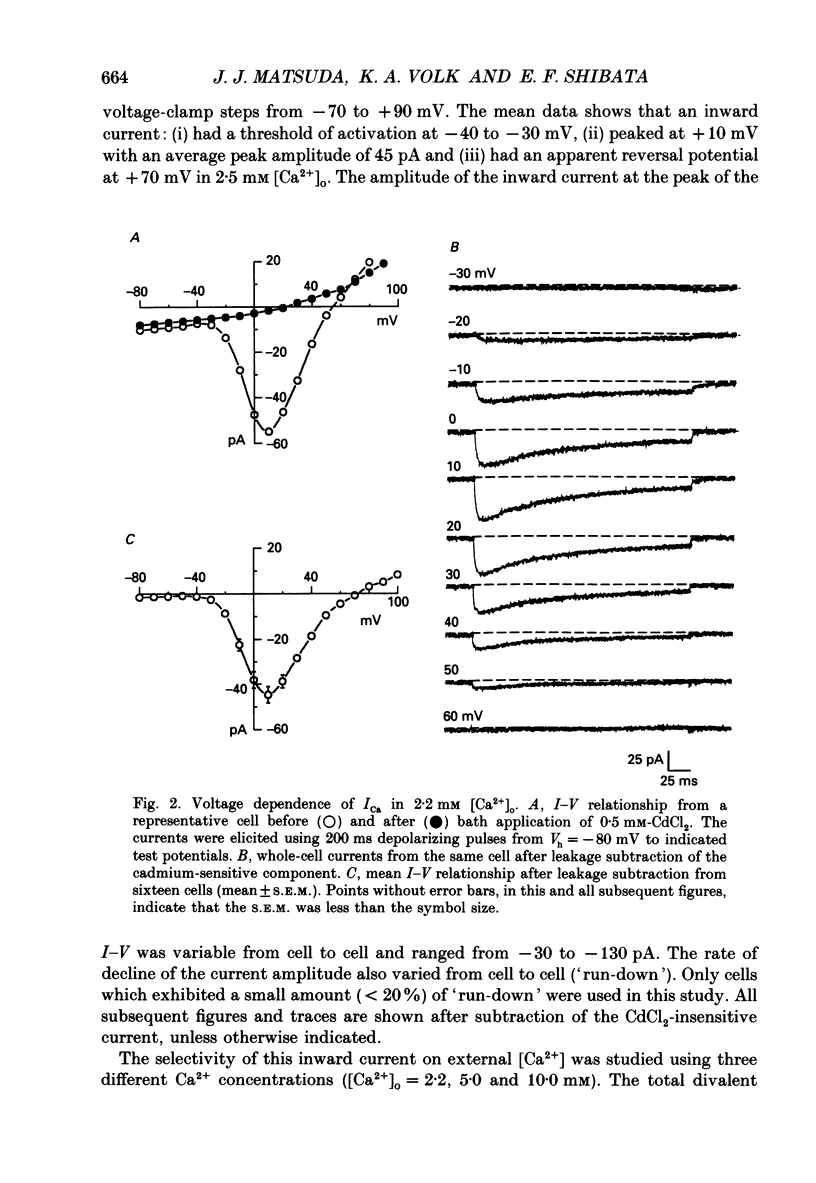
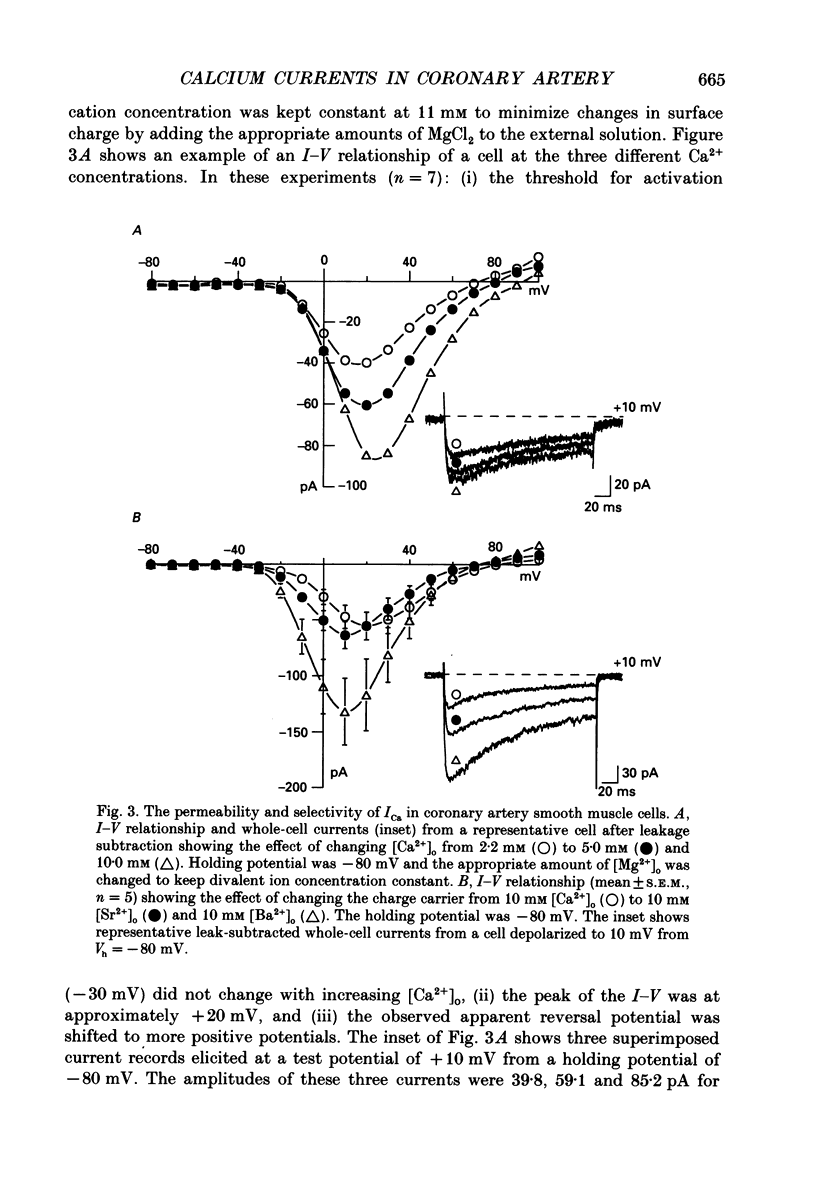
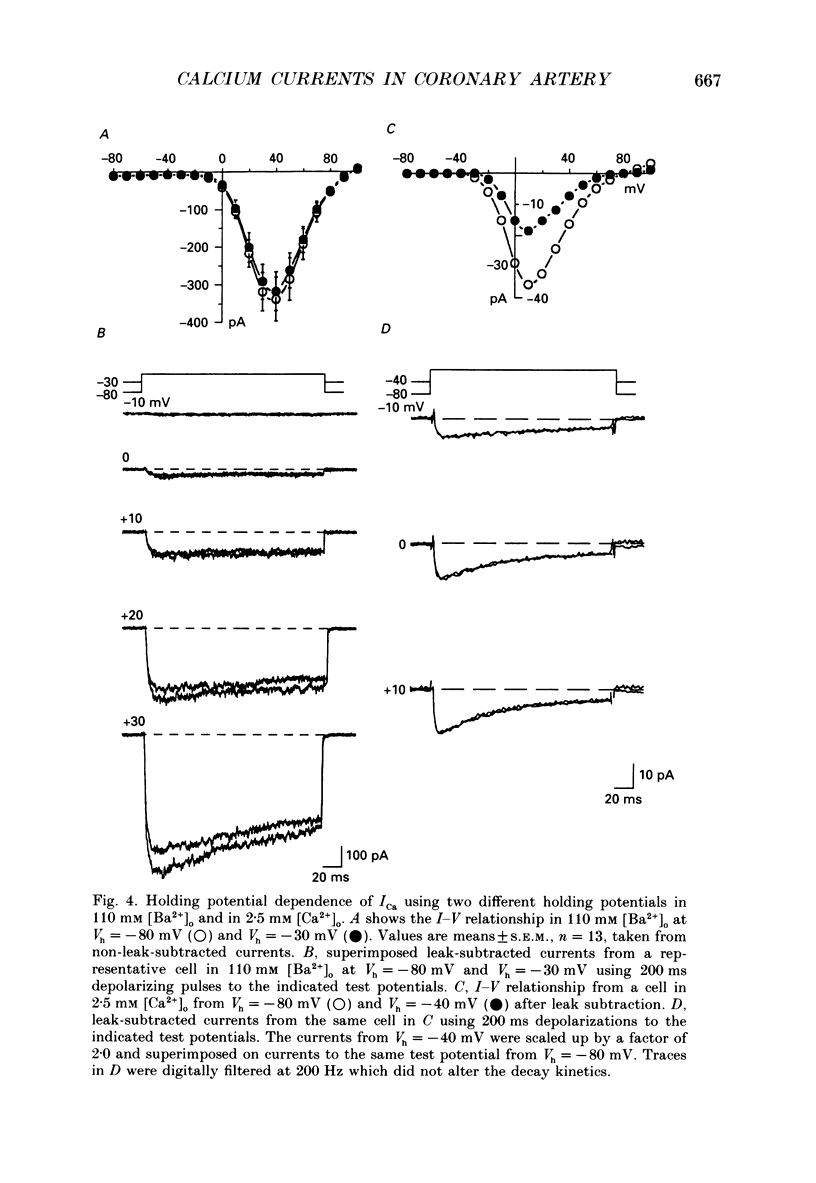
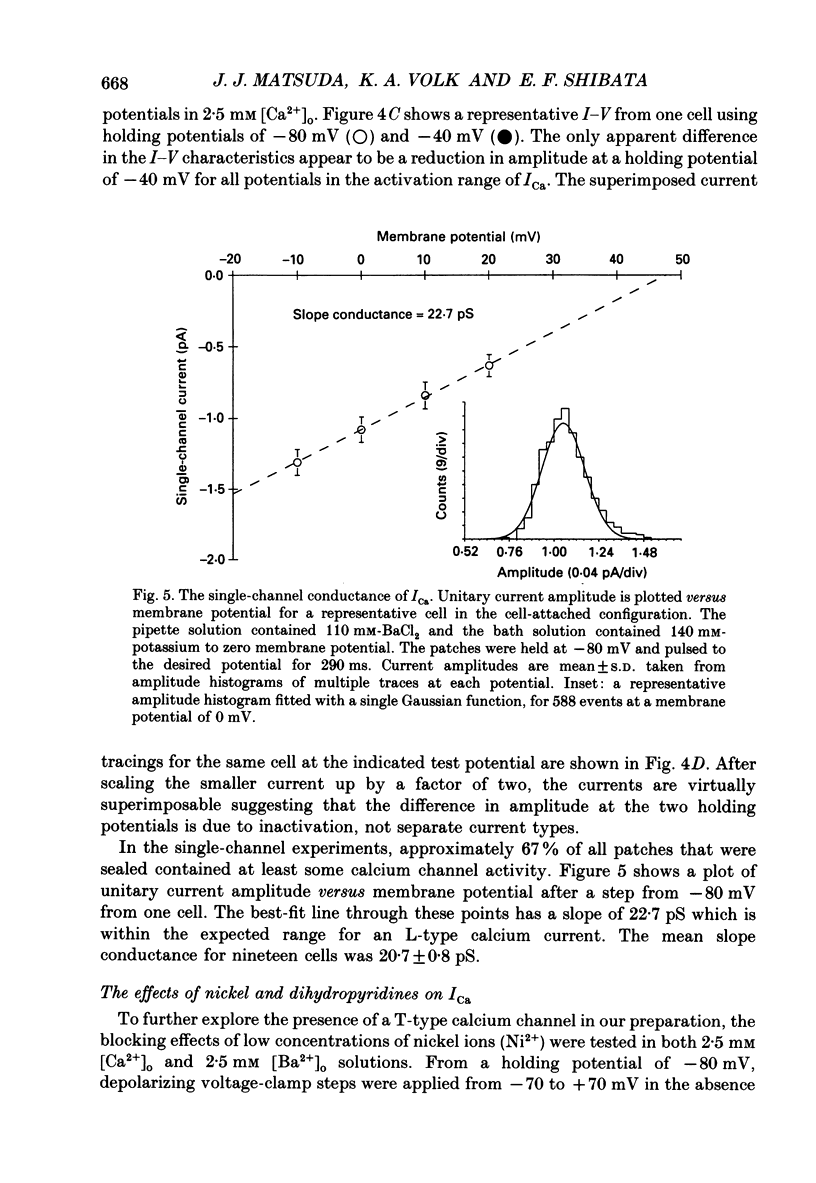
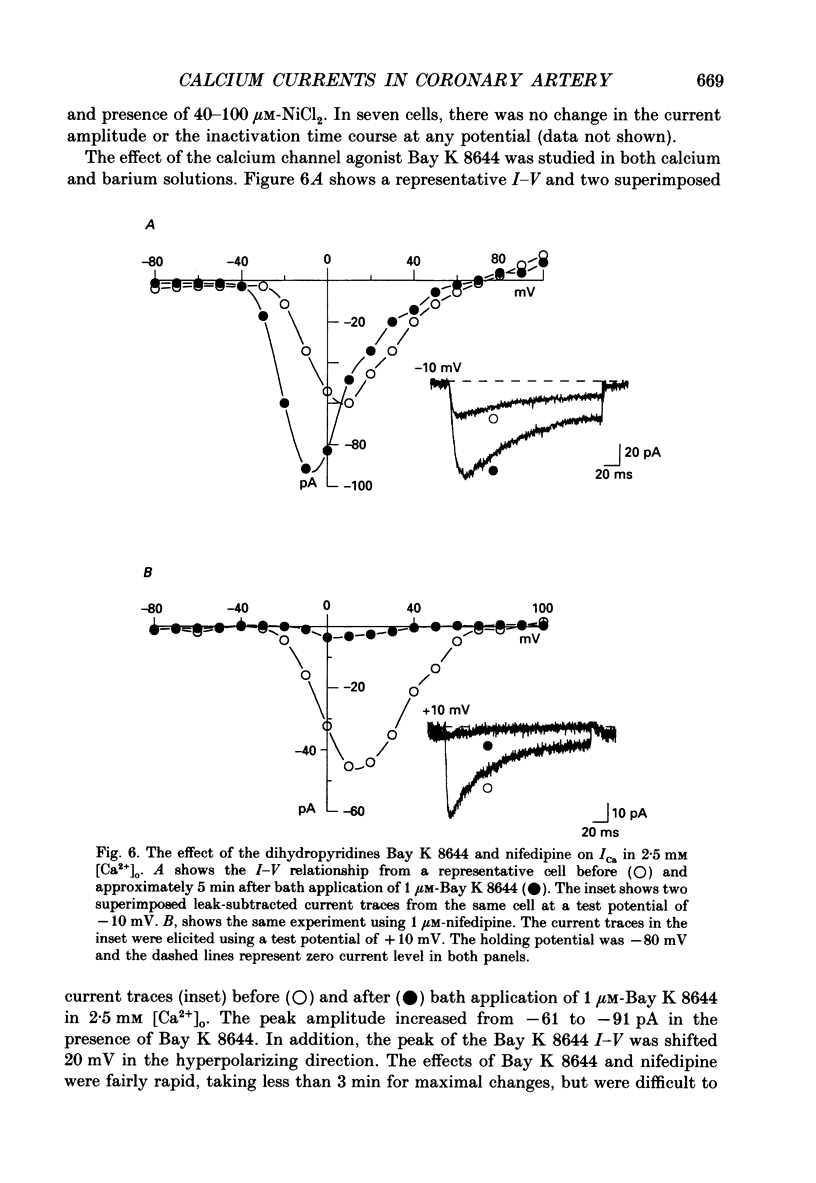
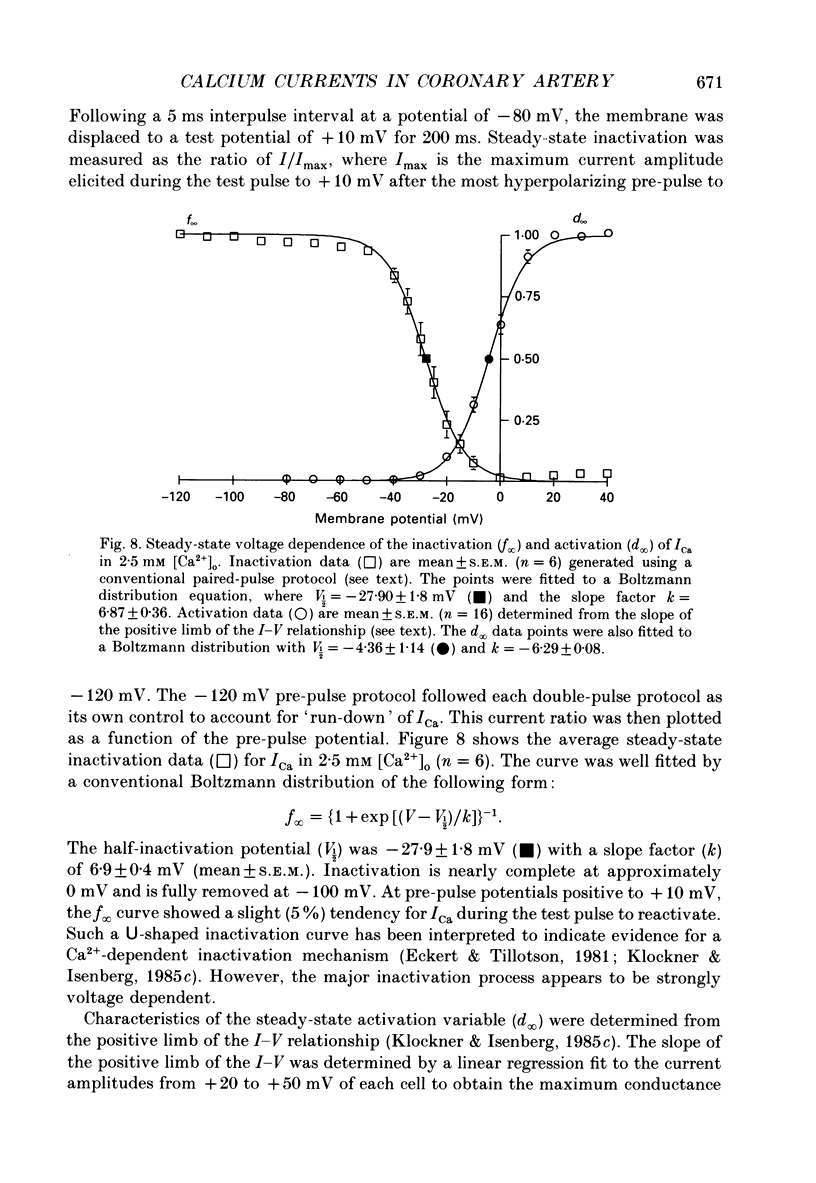
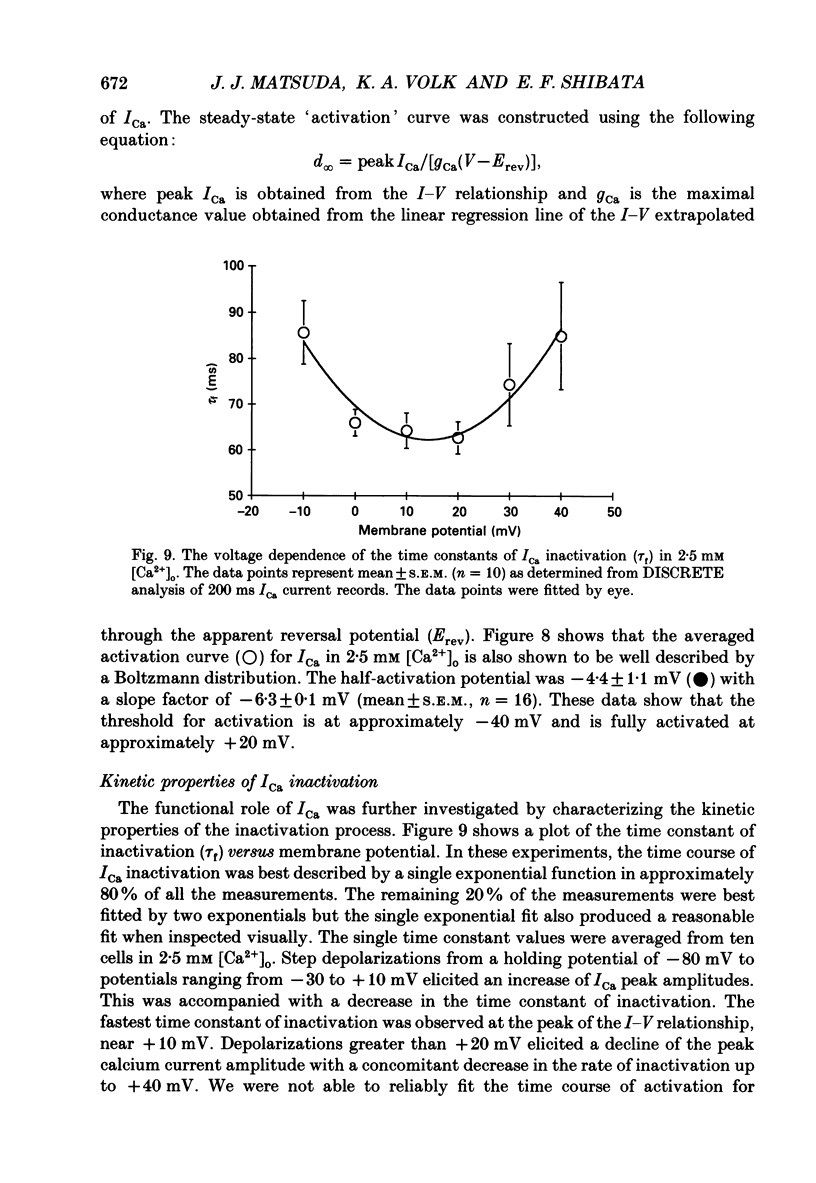
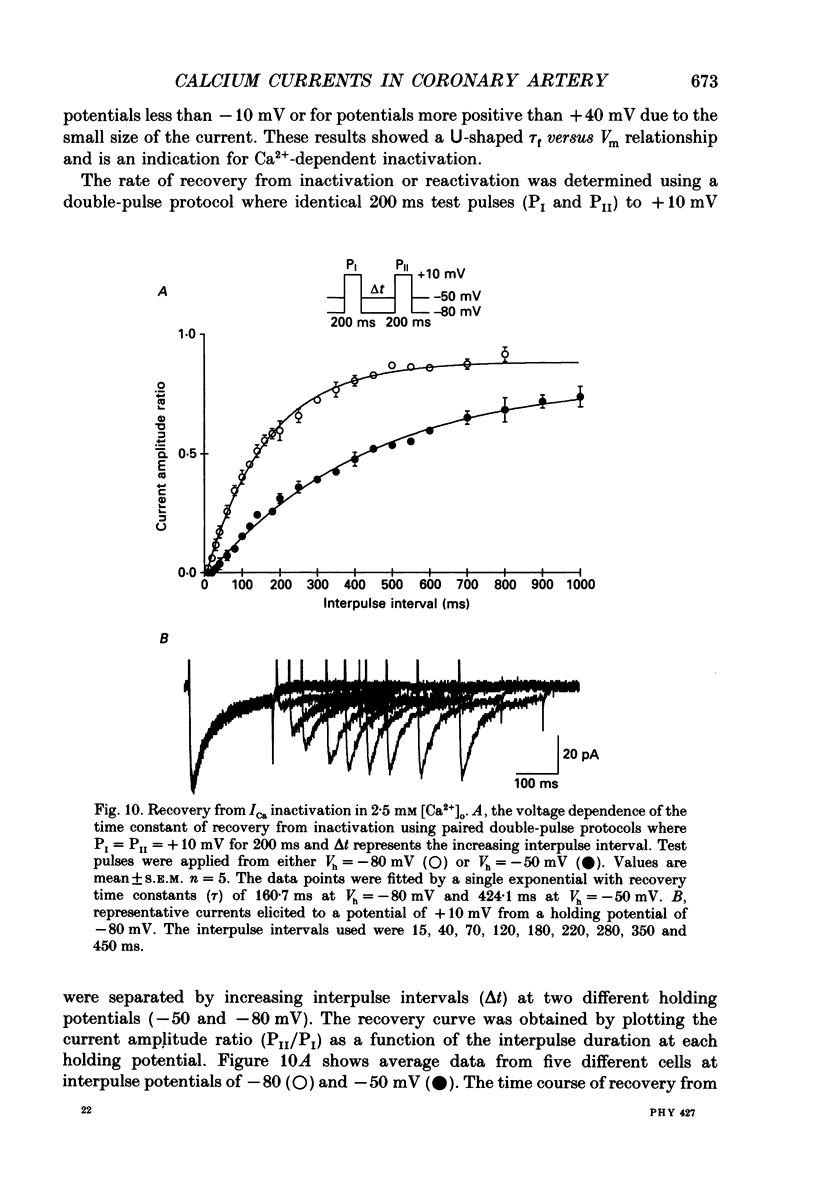
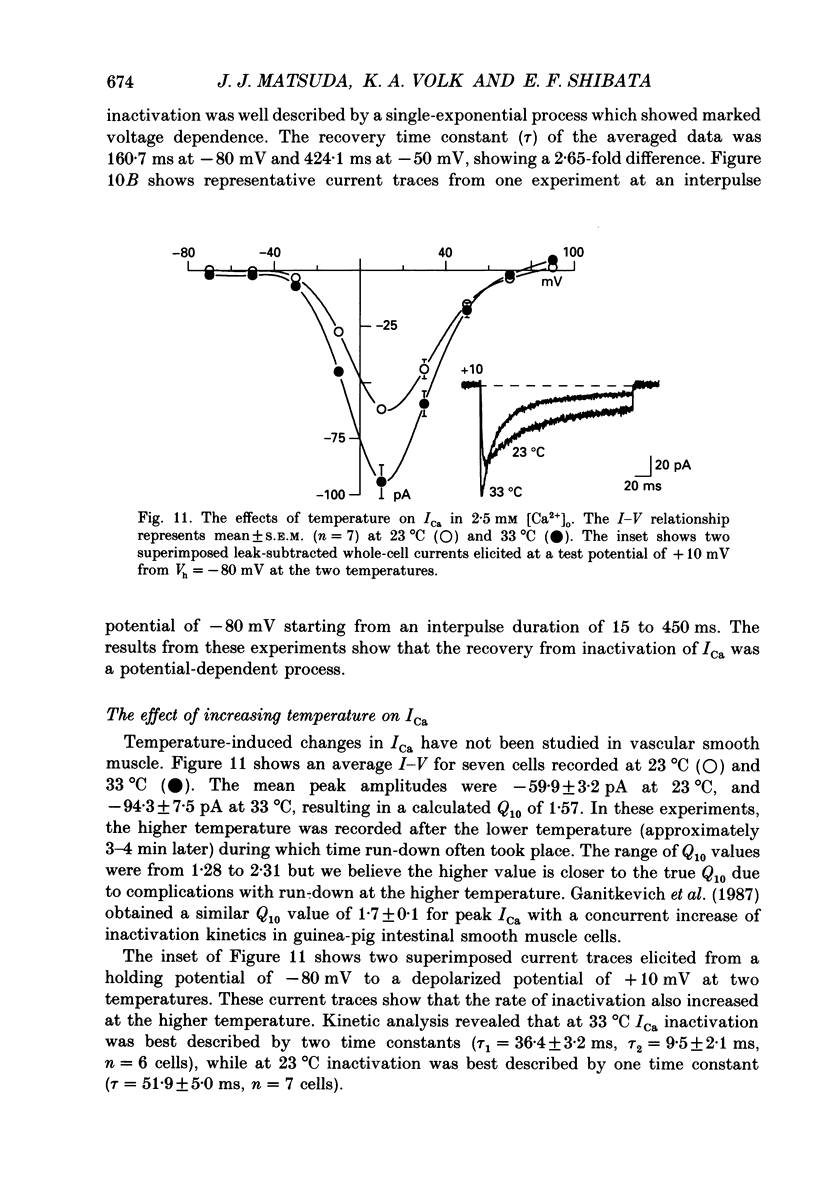
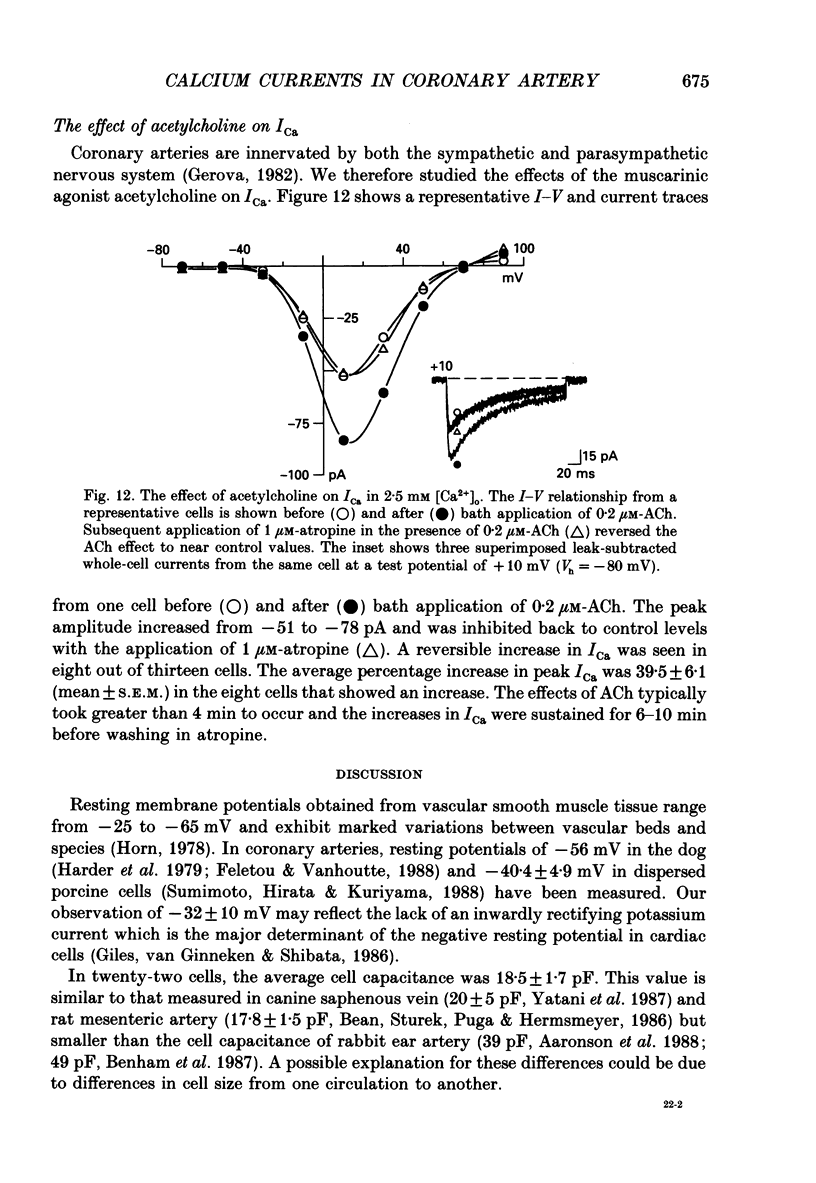
1. Calcium inward currents were recorded from relaxed enzymatically isolated smooth muscle cells from the rabbit epicardial left descending coronary artery using a single-pipette voltage-clamp technique. Outward K+ currents were blocked with CsCl-tetraethylammonium-filled pipette solutions. 2. Relaxed coronary smooth muscle cells had a maximum diameter of 8.6 +/- 0.6 microns and a cell length of 96.7 +/- 3.3 microns when bathed in 2.5 mM [Ca2+]o. The average resting membrane potential at room temperature was -32 +/- 10 mV. The mean cell capacitance was 18.5 +/- 1.7 pF and the input resistance was 3.79 +/- 0.58 G omega. 3. Depolarizing voltage-clamp steps from a holding potential of -80 mV elicited a single time- and voltage-dependent inward current which was dependent upon extracellular [Ca2+]. In 2.5 mM [Ca2+]o, the inward current was activated at a potential of -40 mV and peaked at +10 mV. This current was inhibited by 0.5 mM-CdCl2 and 1 microM-nifedipine and was enhanced with 1 microM-Bay K 8644. No detectable low-threshold, rapidly inactivating T-type calcium current was observed. 4. The apparent reversal potential of this inward current in 2.5 mM [Ca2+]o was +70 mV and shifted by 33.0 mV per tenfold increase in [Ca2+]o. This channel was also more permeable to barium and strontium ions than to calcium ions. 5. Single calcium channel recordings with 110 mM-Ba2+ as the charge carrier revealed a mean slope conductance of 20.7 +/- 0.8 pS. 6. This calcium current (ICa) exhibited a strong voltage-dependent inactivation process. However, the steady-state inactivation curve (f infinity) displayed a slight nonmonotonic, U-shaped dependence upon membrane potential. The potential at which half of the channels were inactivated was -27.9 mV with a slope factor of 6.9 mV. The steady-state activation curve (d infinity) was also well-described by a Boltzmann distribution with a half-activation potential at -4.4 mV and a slope factor of -63 mV. ICa was fully activated at approximately +20 mV. 7. The rate of inactivation was dependent upon the species of ion carrying the current. Both Sr2+ and Ba2+ decreased the rate as well as the degree of inactivation. The tau f (fitted time constant of inactivation) curve displayed a U-shaped relationship in 2.5 mM [Ca2+]o. The reactivation process was voltage dependent and could be described by a single exponential. 8. The current amplitude and the inactivation kinetics were temperature dependent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988 Oct;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Chilian W. M., Eastham C. L., Marcus M. L. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986 Oct;251(4 Pt 2):H779–H788. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Vivaudou M. B., Walsh J. V., Jr, Singer J. J. Acetylcholine increases voltage-activated Ca2+ current in freshly dissociated smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2092–2096. doi: 10.1073/pnas.84.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Declerck I., Casteels R. Effect of adrenergic agonists on Ca2+-channel currents in single vascular smooth muscle cells. Pflugers Arch. 1987 Jun;409(1-2):7–12. doi: 10.1007/BF00584744. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl E. O. Coronary physiology. Physiol Rev. 1983 Jan;63(1):1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Potential-dependent calcium inward current in a single isolated smooth muscle cell of the guinea-pig taenia caeci. J Physiol. 1986 Nov;380:1–16. doi: 10.1113/jphysiol.1986.sp016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Belardinelli L., Sperelakis N., Rubio R., Berne R. M. Differential effects of adenosine and nitroglycerin on the action potentials of large and small coronary arteries. Circ Res. 1979 Feb;44(2):176–182. doi: 10.1161/01.res.44.2.176. [DOI] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Silverberg G. D., van Helden D. F. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986 Feb;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Active and passive electrical properties of single bullfrog atrial cells. J Gen Physiol. 1981 Jul;78(1):19–42. doi: 10.1085/jgp.78.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Cholinergic constriction in the general circulation and its role in coronary artery spasm. Circ Res. 1989 Aug;65(2):237–257. doi: 10.1161/01.res.65.2.237. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological properties of the smooth muscle cell membrane of the dog coronary artery. J Physiol. 1980 Jan;298:205–212. doi: 10.1113/jphysiol.1980.sp013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto K., Hirata M., Kuriyama H. Characterization of [3H]nifedipine binding to intact vascular smooth muscle cells. Am J Physiol. 1988 Jan;254(1 Pt 1):C45–C52. doi: 10.1152/ajpcell.1988.254.1.C45. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Yamazawa T., Harada Y., Yamamura H. I., Nakayama K. Muscarinic receptor subtype in porcine coronary artery. Eur J Pharmacol. 1988 Jun 10;150(3):373–376. doi: 10.1016/0014-2999(88)90021-0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- de la Lande I., Harvey J. A., Holt S. Response to the rabbit coronary arteries to autonomic agents. Blood Vessels. 1974;11(5-6):319–337. doi: 10.1159/000158025. [DOI] [PubMed] [Google Scholar]


