Abstract
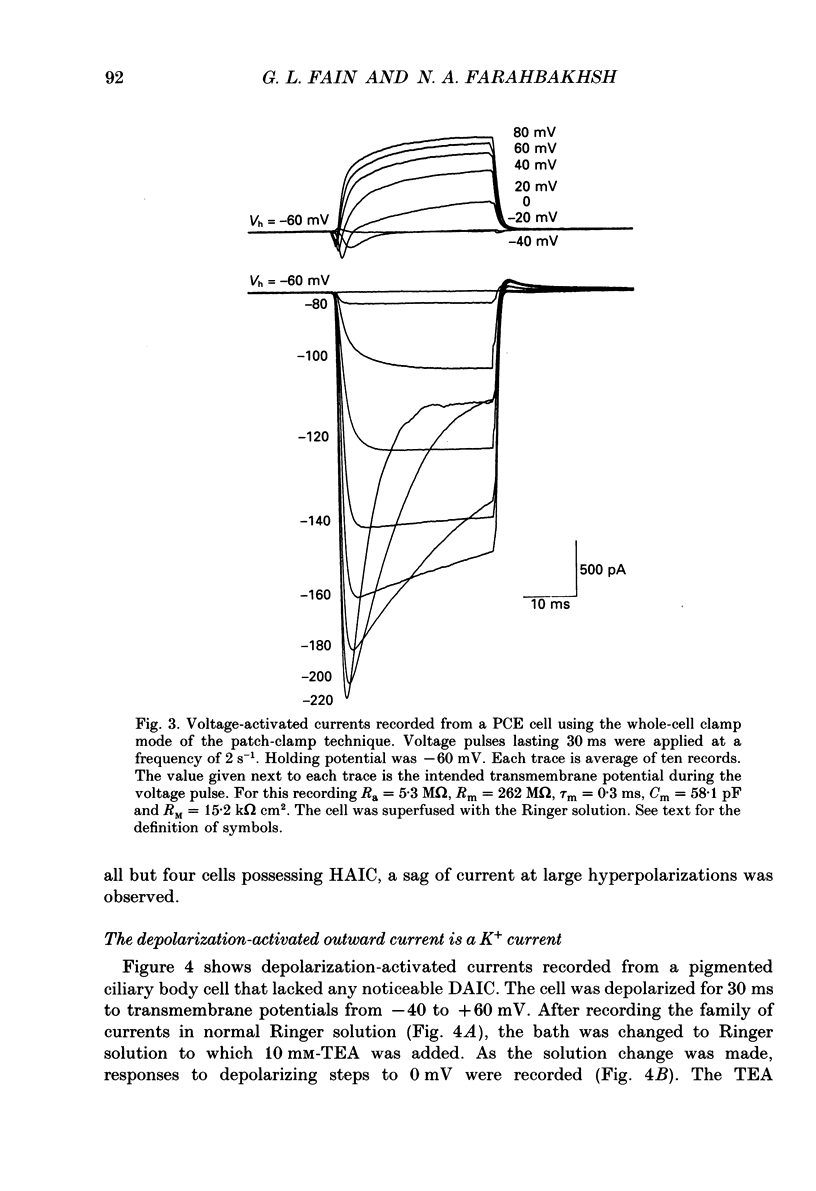
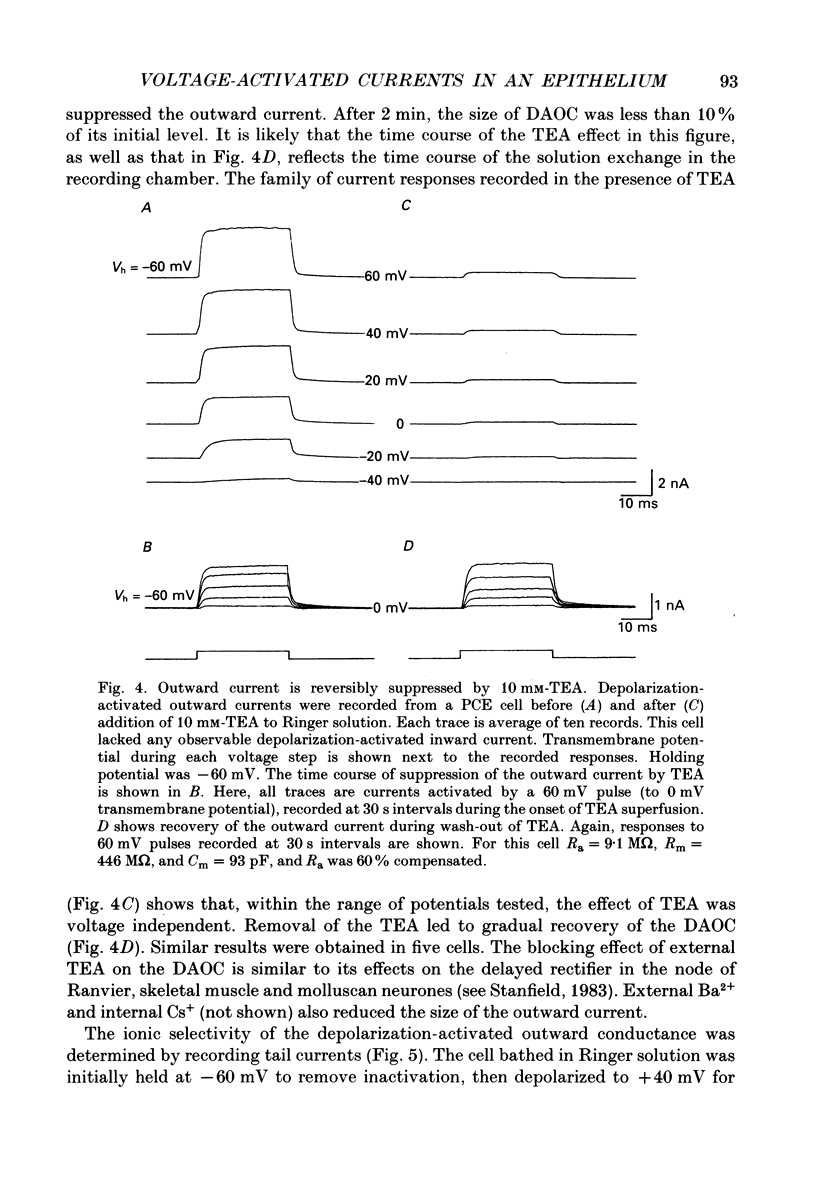
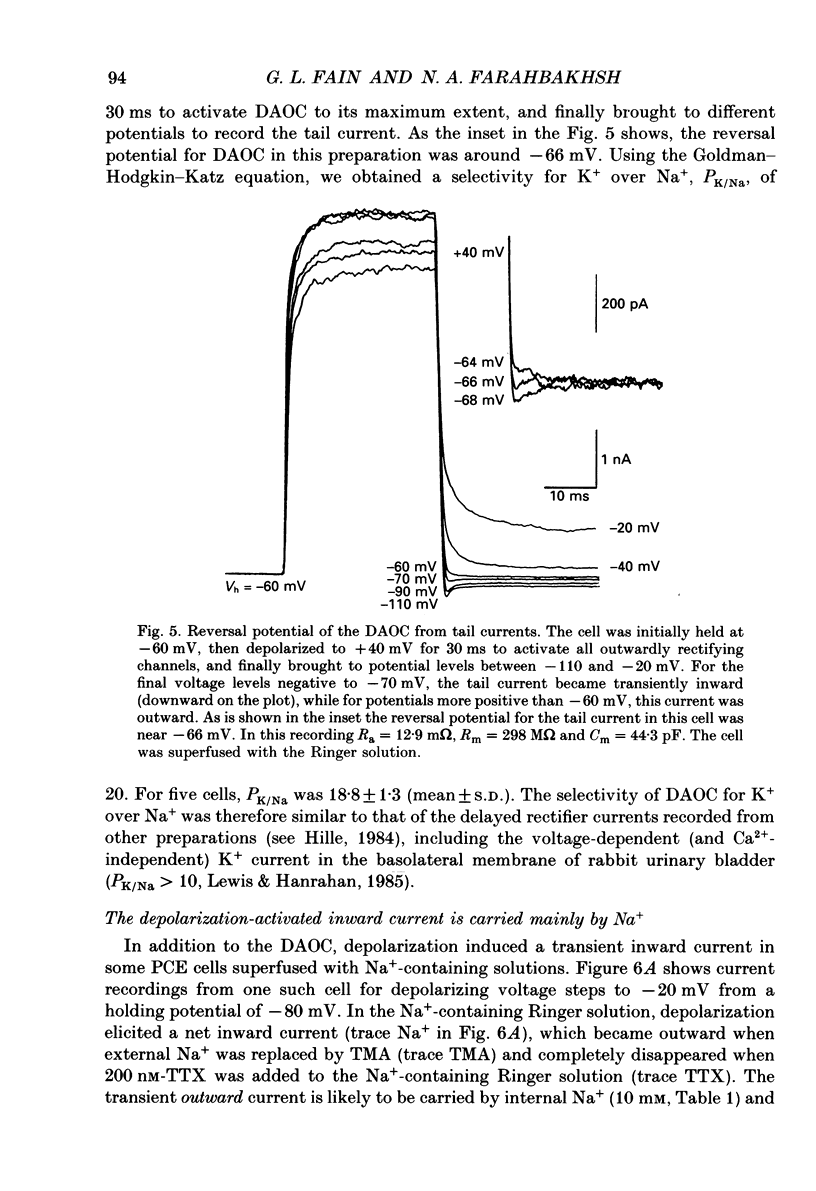
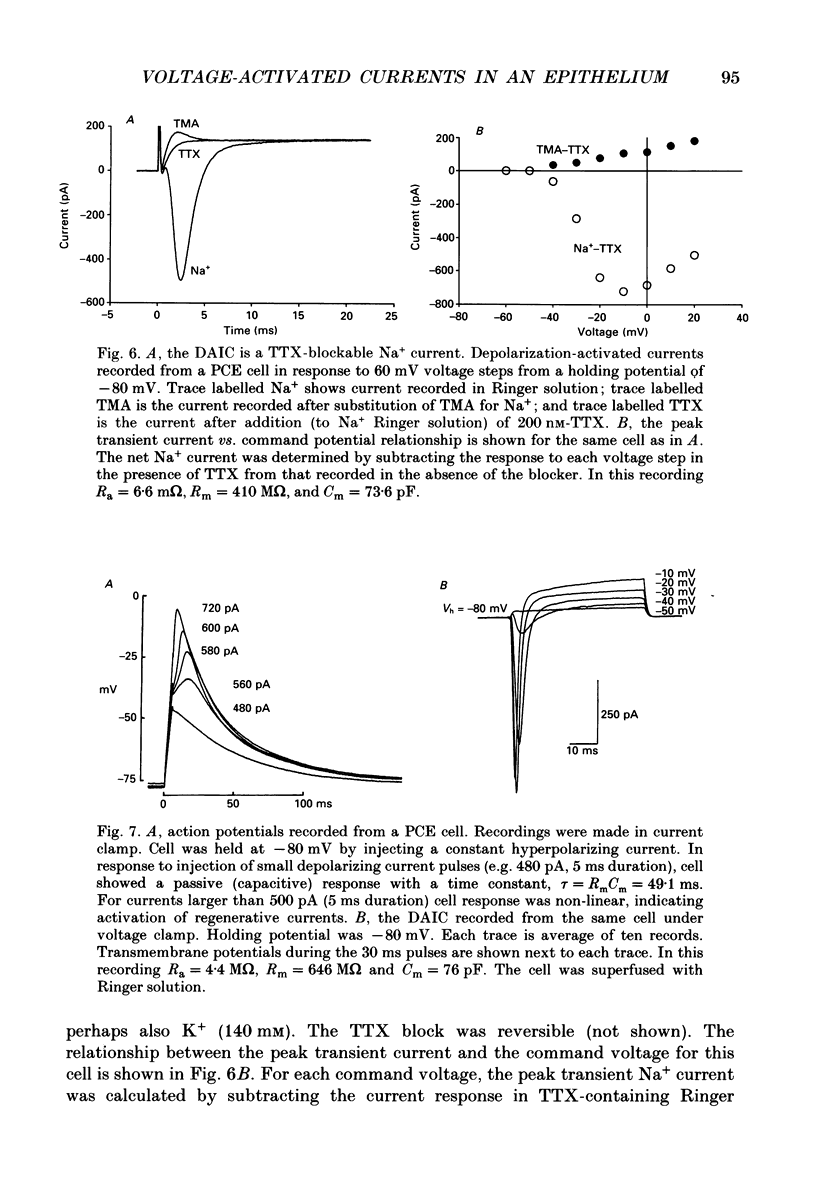
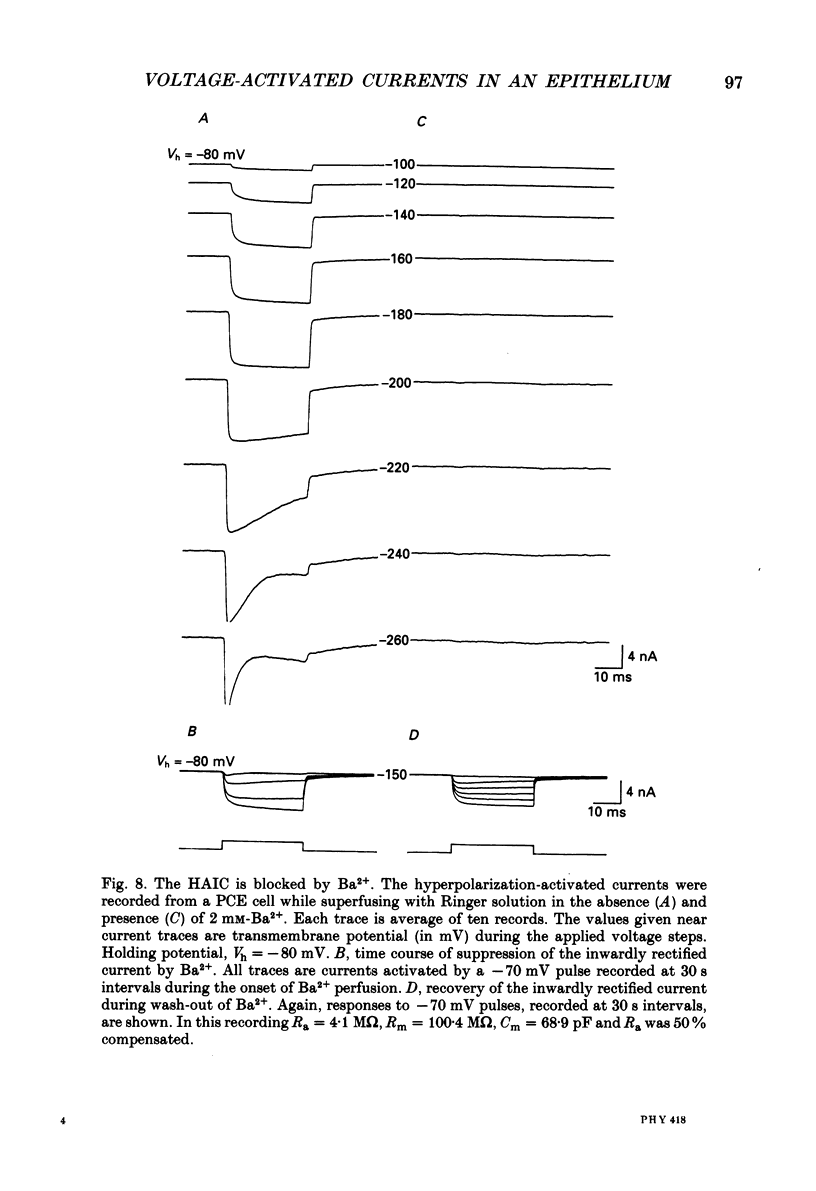
1. The whole-cell recording mode of the patch-clamp technique was used to investigate the presence of voltage-activated currents in the isolated pigmented cells from the rabbit ciliary body epithelium grown in culture. 2. In Ringer solution with composition similar to that of the rabbit aqueous humour, depolarizing voltage steps activated a transient inward current and a delayed outward current, while hyperpolarization elicited an inwardly rectified current. 3. The depolarization-activated inward current was mainly carried by Na+ and was blocked by submicromolar concentrations of tetrodotoxin. This current in many cells was sufficiently large to produce a regenerative Na+ spike. 4. The depolarization-activated outward current was carried by K+ and blocked by external TEA and Ba2+. Its activation appeared to be Ca2(+)-independent. 5. The hyperpolarization-activated inward current was almost exclusively carried by K+ and was blocked by Ba2+ and Cs+. For large hyperpolarizations below -120 mV, this current exhibited a biphasic activation with a fast transient peak followed by a slower sag, that appeared to be due to K+ depletion. 6. The voltage-dependent K+ conductances probably act to stabilize the cell membrane resting potential and may also play a role in ion transport. The function of the Na(+)-dependent inward current is unclear, but it may permit the electrically coupled epithelial cells of the ciliary body to conduct propagated action potentials.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. A. Epithelial conduction: its properties and function. Prog Neurobiol. 1980;15(3):161–203. doi: 10.1016/0301-0082(80)90022-2. [DOI] [PubMed] [Google Scholar]
- Avenet P., Lindemann B. Patch-clamp study of isolated taste receptor cells of the frog. J Membr Biol. 1987;97(3):223–240. doi: 10.1007/BF01869225. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C., Anand-Srivastava M. B., De Léan A., Gutkowska J., Forthomme D., Genest J., Cantin M. Localization and characterization of specific receptors for atrial natriuretic factor in the ciliary processes of the eye. Curr Eye Res. 1986 Apr;5(4):283–293. doi: 10.3109/02713688609020054. [DOI] [PubMed] [Google Scholar]
- Bill A. The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp Eye Res. 1973 Aug 10;16(4):287–298. doi: 10.1016/0014-4835(73)90094-8. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Fritsch J. Voltage-gated sodium and calcium currents in rat osteoblasts. J Physiol. 1988 Apr;398:291–311. doi: 10.1113/jphysiol.1988.sp017043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T. C., Candia O. A., Iizuka S. Effects of forskolin, prostaglandin F2 alpha, and Ba2+ on the short-circuit current of the isolated rabbit iris-ciliary body. Curr Eye Res. 1986 Jul;5(7):511–516. doi: 10.3109/02713688608996373. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Smolka A., Cilluffo M. C., Fain M. J., Lee D. A., Brecha N. C., Sachs G. Monoclonal antibodies to the H+-K+ ATPase of gastric mucosa selectively stain the non-pigmented cells of the rabbit ciliary body epithelium. Invest Ophthalmol Vis Sci. 1988 May;29(5):785–794. [PubMed] [Google Scholar]
- Farahbakhsh N. A., Fain G. L. Volume regulation of non-pigmented cells from ciliary epithelium. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):934–944. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
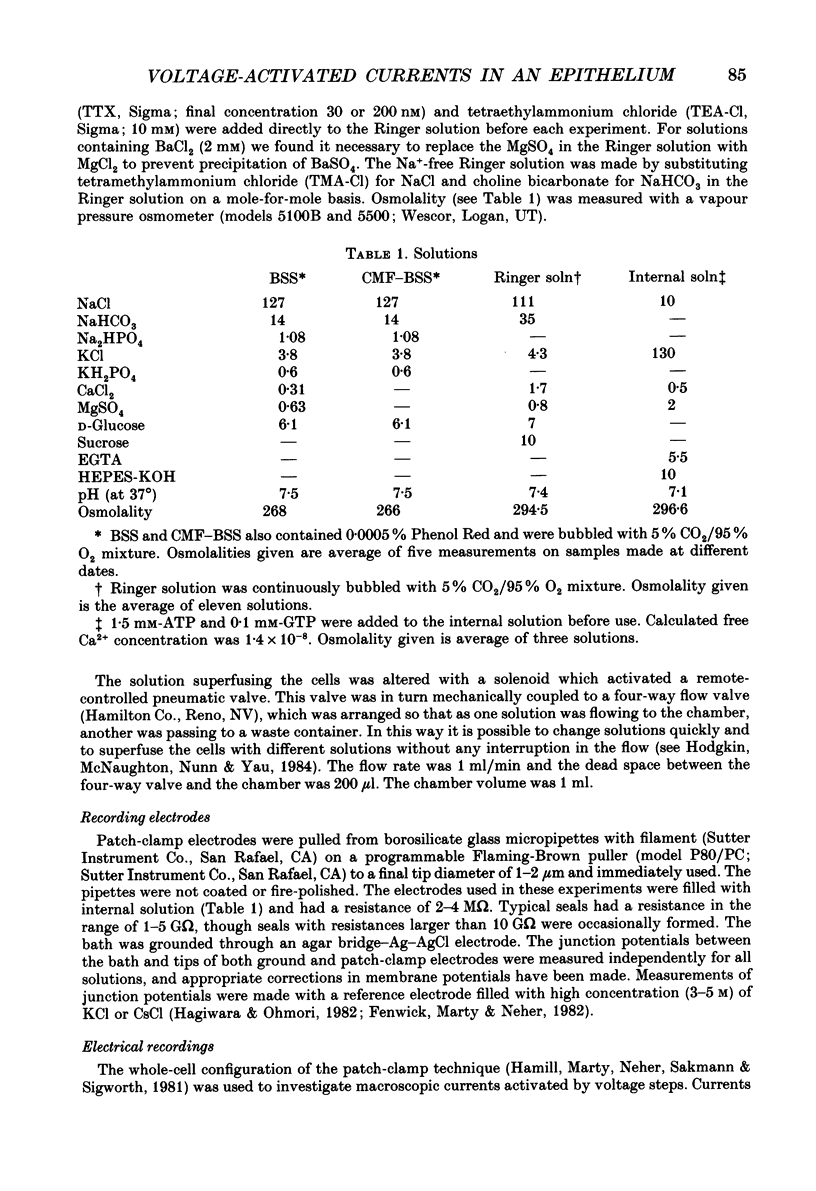
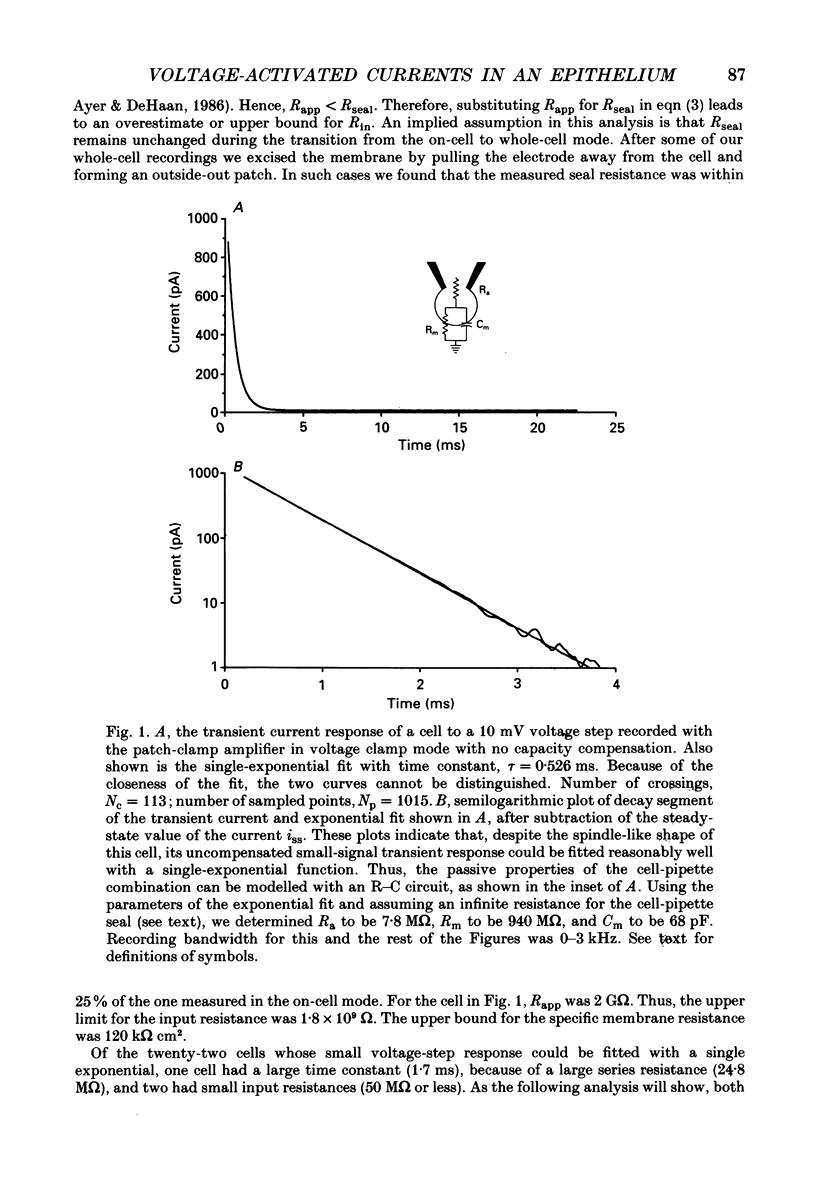
- Fischmeister R., Ayer R. K., Jr, DeHaan R. L. Some limitations of the cell-attached patch clamp technique: a two-electrode analysis. Pflugers Arch. 1986 Jan;406(1):73–82. doi: 10.1007/BF00582957. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., McKinney L. C. Potassium conductances in macrophages. Soc Gen Physiol Ser. 1988;43:315–332. [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Green K., Bountra C., Georgiou P., House C. R. An electrophysiologic study of rabbit ciliary epithelium. Invest Ophthalmol Vis Sci. 1985 Mar;26(3):371–381. [PubMed] [Google Scholar]
- Green R., Greenwood S. L., White S. The effects of anions on fluid reabsorption from the proximal convoluted tubule of the rat. J Physiol. 1988 Dec;407:103–116. doi: 10.1113/jphysiol.1988.sp017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Helbig H., Korbmacher C., Wiederholt M. K+-conductance and electrogenic Na+/K+ transport of cultured bovine pigmented ciliary epithelium. J Membr Biol. 1987;99(3):173–186. doi: 10.1007/BF01995698. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M., Kawahara K., Giebisch G. Potassium channels along the nephron. Fed Proc. 1986 Nov;45(12):2723–2726. [PubMed] [Google Scholar]
- Hunter M., Oberleithner H., Henderson R. M., Giebisch G. Whole-cell potassium currents in single early distal tubule cells. Am J Physiol. 1988 Oct;255(4 Pt 2):F699–F703. doi: 10.1152/ajprenal.1988.255.4.F699. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Moore J. W., Ramón F. Axon voltage-clamp simulations. III. Postsynaptic region. Biophys J. 1975 Jan;15(1):37–54. doi: 10.1016/S0006-3495(75)85790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G., Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978 Oct;17(10):958–981. [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel gating currents. Origin of the rising phase. J Gen Physiol. 1987 Apr;89(4):521–540. doi: 10.1085/jgp.89.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Attwell D., Mobbs P., Wilson M. Membrane currents in retinal bipolar cells of the axolotl. J Gen Physiol. 1988 Jan;91(1):49–72. doi: 10.1085/jgp.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]



