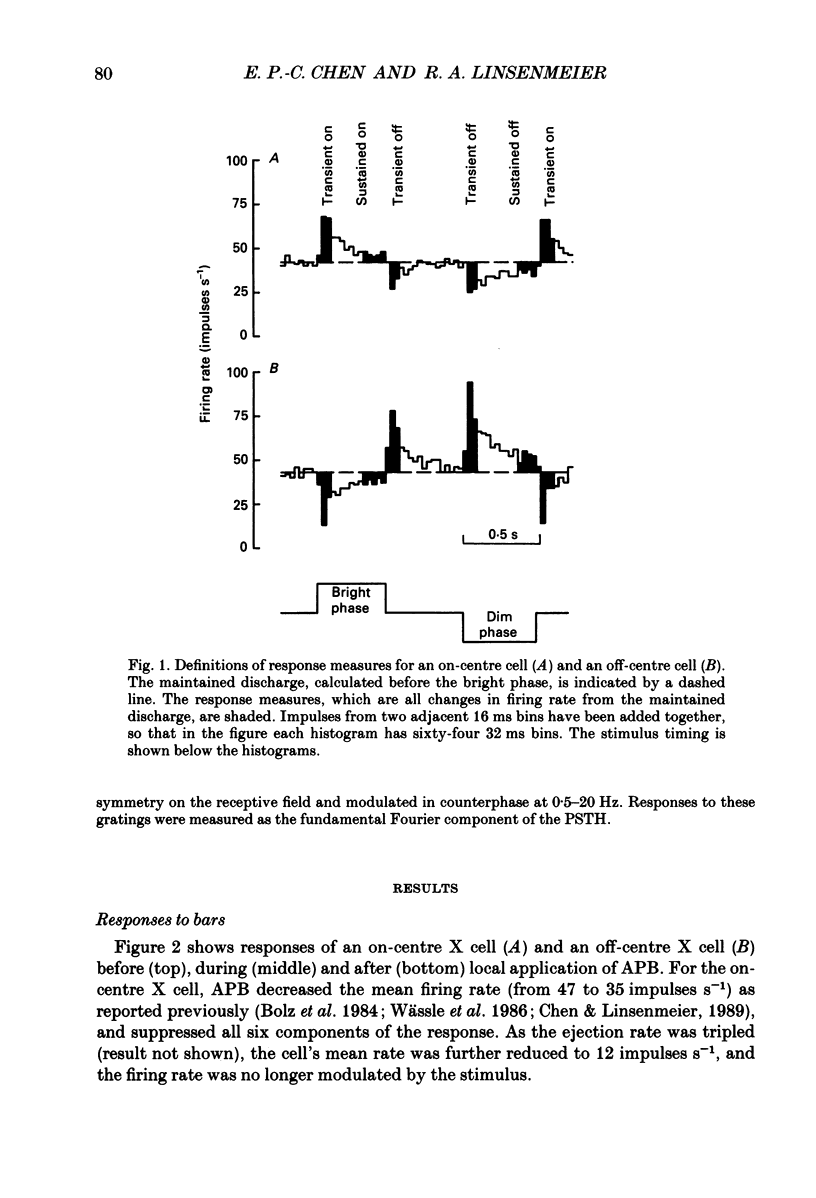
Abstract
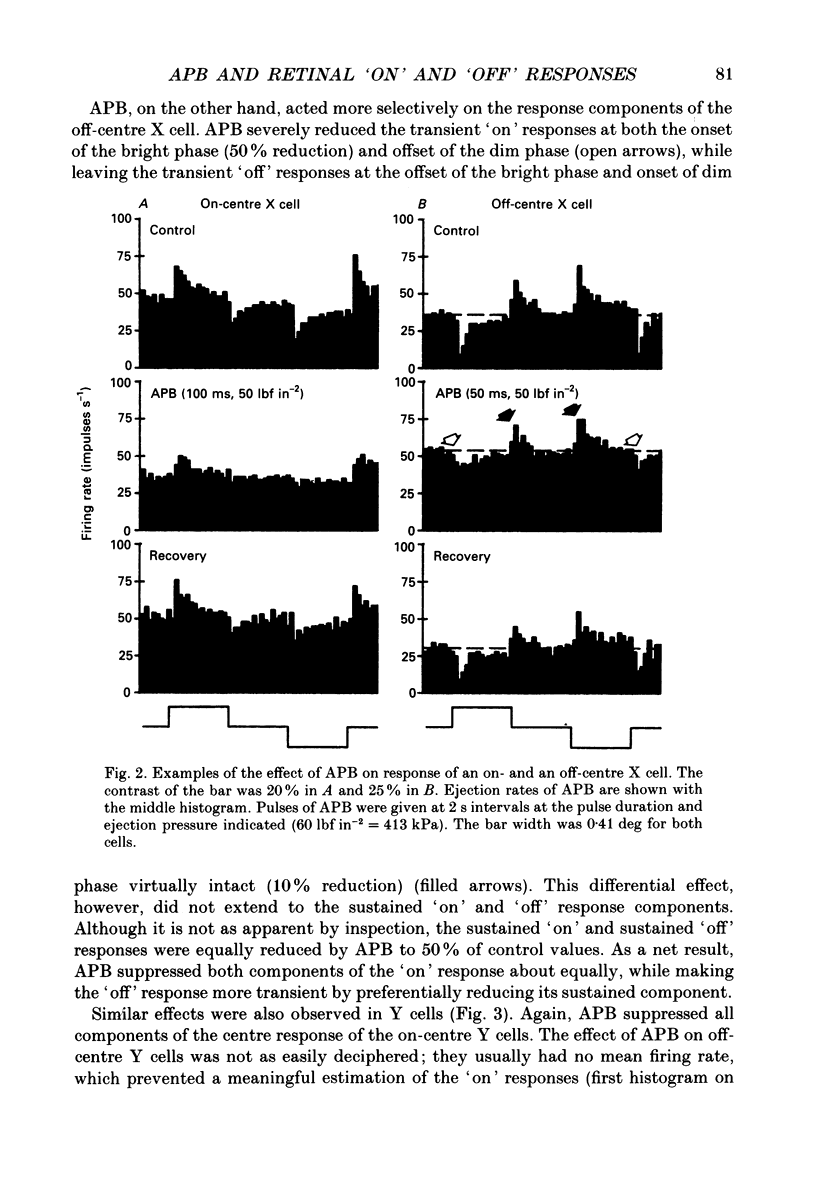
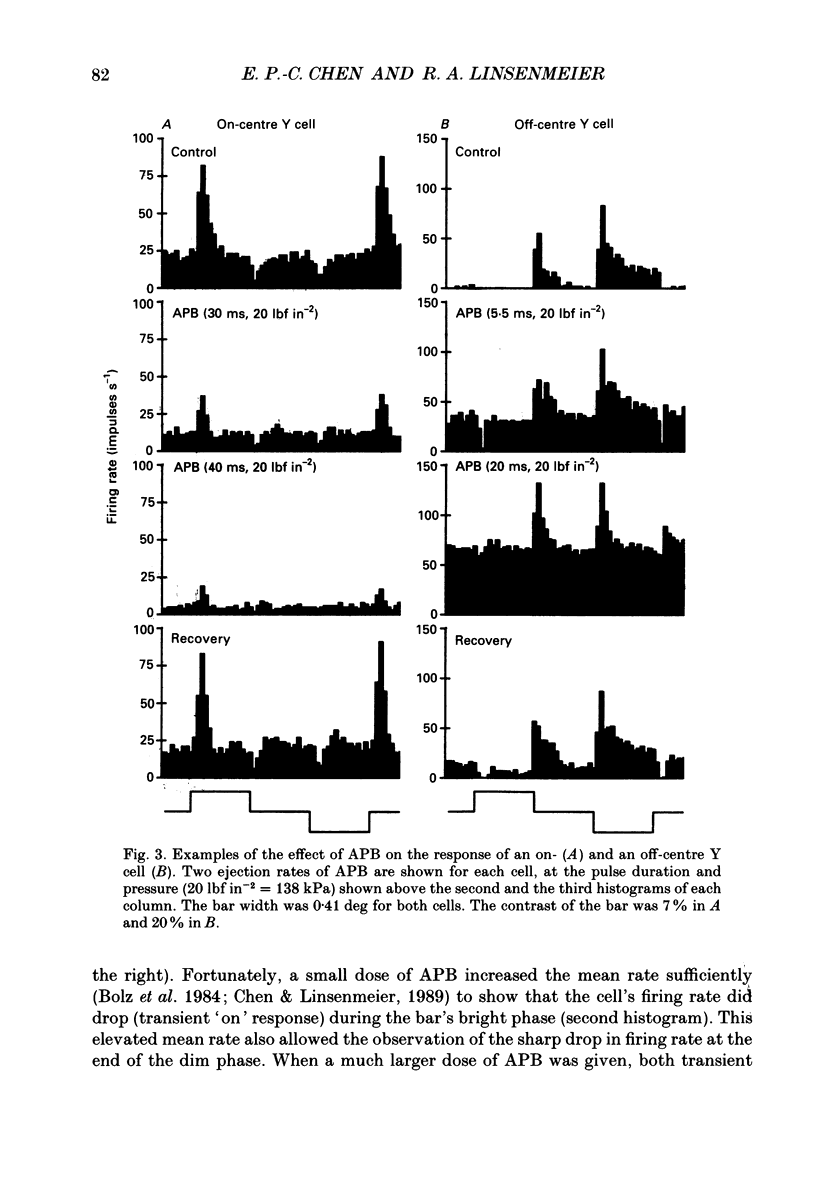
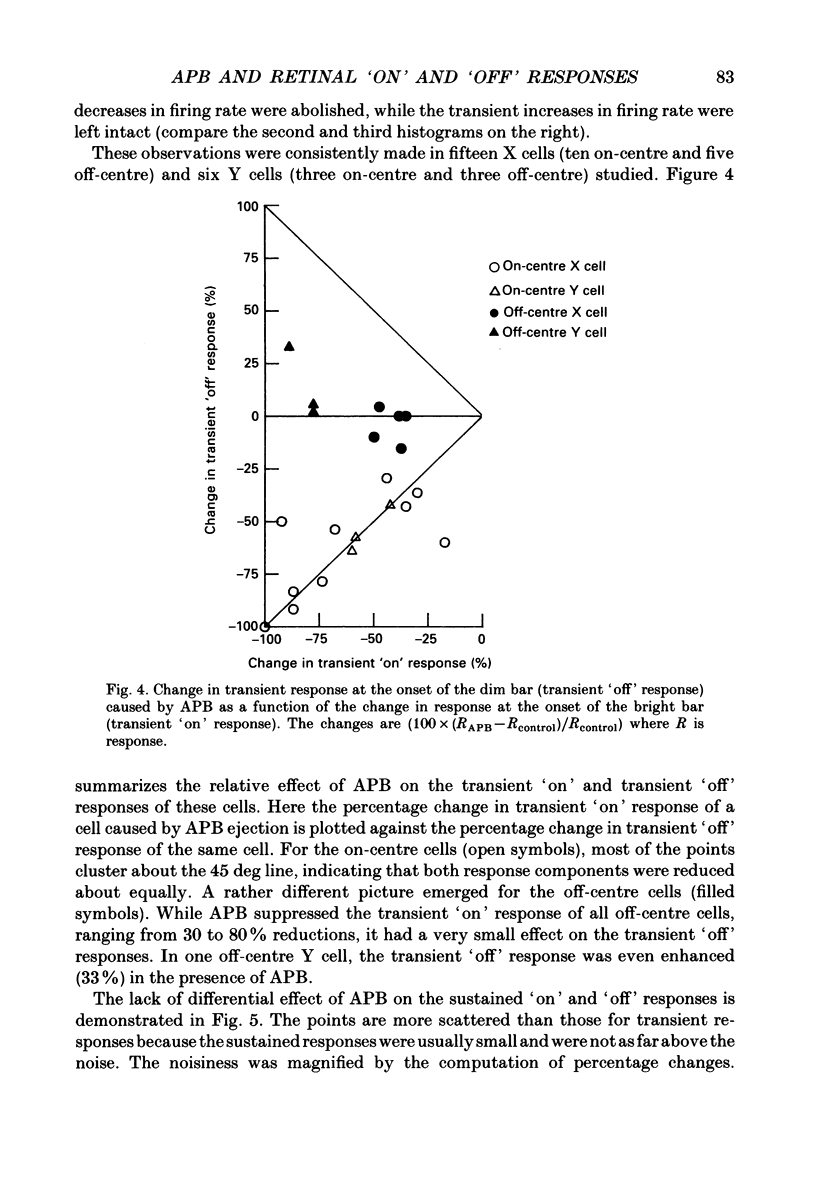
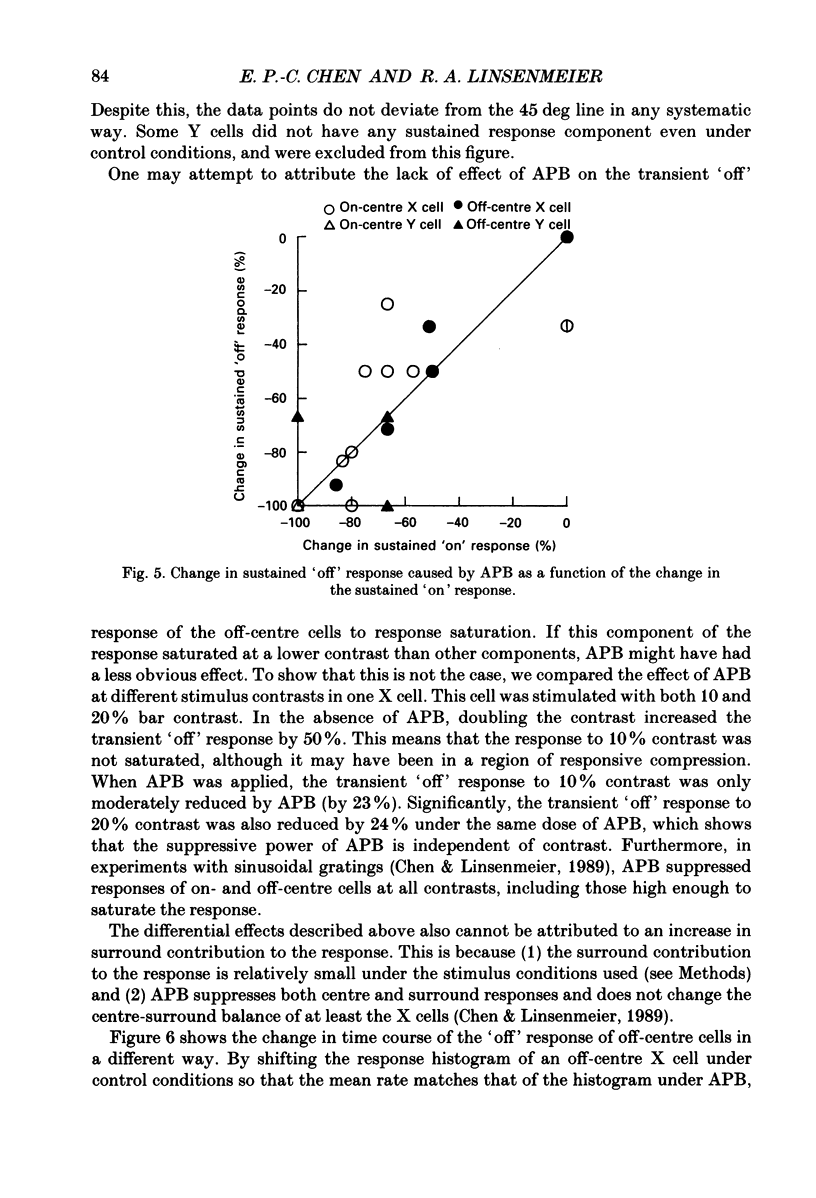
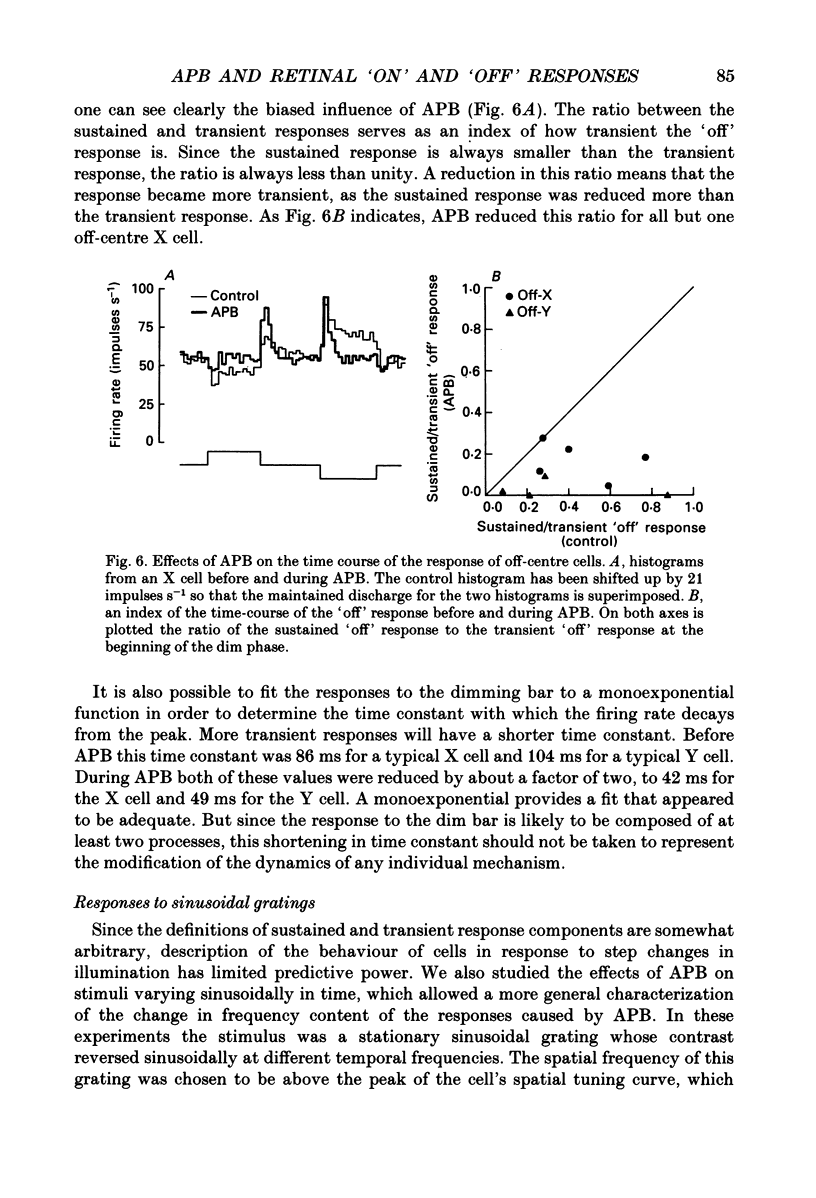
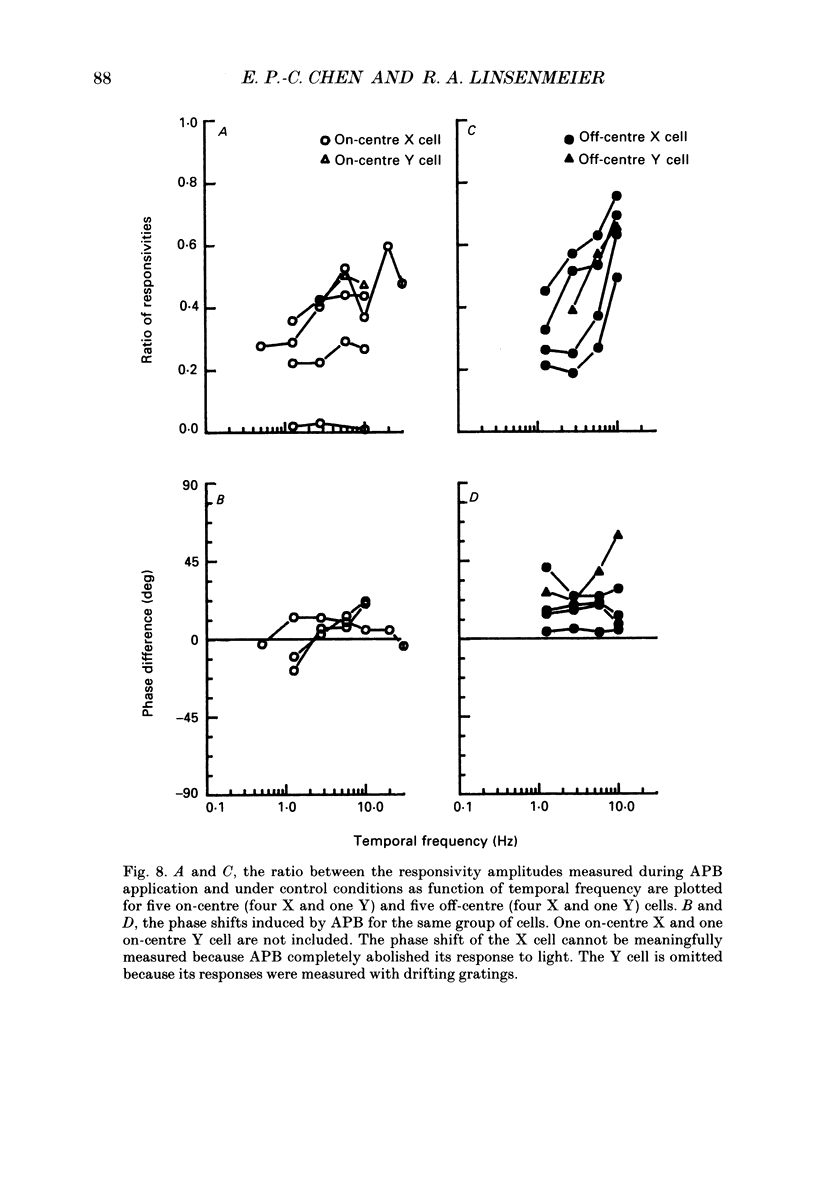
1. Changes in different components of the cone-driven centre responses of cat retinal ganglion cells were studied before and during local application of 2-amino-4-phosphonobutyric acid (APB). Responses were elicited with bar stimuli whose luminance was above ('brighter') or below ('dimmer') the photopic background luminance. The bars were centrally located, and were similar in width to the receptive field centre. 2. APB acted differently on the on- and off-centre cells. For on-centre X and Y cells, all components of the responses to bright and dim bars were diminished by APB. For the off-centre X and Y cells. APB reduced all components except the transient increase in firing rate when the bright bar was turned off or when the dim bar was turned on. 3. These results suggest that the centre response mechanism of off-centre X and Y cells comprises APB-sensitive and APB-resistant components. The APB-sensitive component is more sustained and responds to both brightening and dimming stimuli. The APB-resistant component is more transient and responds primarily to dimming stimuli. For on-centre X and Y cells only APB-sensitive components could be demonstrated. 4. Experiments with stationary sinusoidal gratings modulated at 0.5-10 Hz showed that responses of off-centre cells were more affected by APB at low than at high temporal frequencies, confirming that the APB-sensitive pathway is responsible for more of the low temporal frequency responses. As expected from the responses to bar stimuli, APB had a uniform effect at all temporal frequencies in on-centre cells. 5. For off-centre cells, the APB-sensitive component is probably derived from input from depolarizing bipolar cells, and the APB-resistant component is derived from hyperpolarizing bipolar input, although one or both pathways could also involve amacrine cells. The combination of these pathways increases the range of temporal frequencies to which the cell can respond and also increases the range of response amplitudes. 6. The lack of differential effects on on-centre cells may have several explanations. The most likely explanations are that only depolarizing bipolars contribute significantly to the centre responses of these cells under the conditions of these experiments, or that there is an APB-sensitive synapse somewhere in the retina besides the one from cones to depolarizing bipolars.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amthor F. R., Wolbarsht M. L., Ringo J. L. Functional implications of On-Off response variation in frog retinal ganglion cells. Vision Res. 1983;23(1):21–32. doi: 10.1016/0042-6989(83)90037-8. [DOI] [PubMed] [Google Scholar]
- Arkin M. S., Miller R. F. Bipolar origin of synaptic inputs to sustained OFF-ganglion cells in the mudpuppy retina. J Neurophysiol. 1988 Sep;60(3):1122–1142. doi: 10.1152/jn.1988.60.3.1122. [DOI] [PubMed] [Google Scholar]
- Arkin M. S., Miller R. F. Synaptic inputs and morphology of sustained ON-ganglion cells in the mudpuppy retina. J Neurophysiol. 1988 Sep;60(3):1143–1159. doi: 10.1152/jn.1988.60.3.1143. [DOI] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S. Sustained synaptic input to ganglion cells of mudpuppy retina. J Physiol. 1982 May;326:91–108. doi: 10.1113/jphysiol.1982.sp014179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield S. A., Dowling J. E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol. 1985 Mar;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- Bolz J., Wässle H., Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984 Jul;12(3):875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Chen E. P., Linsenmeier R. A. Effects of 2-amino-4-phosphonobutyric acid on responsivity and spatial summation of X cells in the cat retina. J Physiol. 1989 Dec;419:59–75. doi: 10.1113/jphysiol.1989.sp017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Sterling P. Accumulation of (3H)glycine by cone bipolar neurons in the cat retina. J Comp Neurol. 1986 Aug 1;250(1):1–7. doi: 10.1002/cne.902500102. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Pure central responses from off-centre cells and pure surround responses from on-centre cells. J Physiol. 1972 Jan;220(2):441–464. doi: 10.1113/jphysiol.1972.sp009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed M. A., Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988 Jul;8(7):2303–2320. doi: 10.1523/JNEUROSCI.08-07-02303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Trichromatic mechanisms in single cortical neurons. Science. 1970 Apr 24;168(3930):489–492. doi: 10.1126/science.168.3930.489. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Sherk H. Receptive field properties in the cat's lateral geniculate nucleus in the absence of on-center retinal input. J Neurosci. 1984 Feb;4(2):374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. Variability in ganglion cell firing patterns; implications for separate "on" and "off" processes. Vision Res. 1977;17(7):765–776. doi: 10.1016/0042-6989(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Frishman L. J., Jakiela H. G., Enroth-Cugell C. Receptive field properties of x and y cells in the cat retina derived from contrast sensitivity measurements. Vision Res. 1982;22(9):1173–1183. doi: 10.1016/0042-6989(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P. D., McReynolds J. S. Synaptic transmission at N-methyl-D-aspartate receptors in the proximal retina of the mudpuppy. J Physiol. 1985 Oct;367:99–115. doi: 10.1113/jphysiol.1985.sp015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Stevens J. K., Sterling P. Microcircuitry of beta ganglion cells in cat retina. J Neurosci. 1986 Apr;6(4):907–918. doi: 10.1523/JNEUROSCI.06-04-00907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Stevens J. K., Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984 Dec;4(12):2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Wässle H., Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol. 1988 Jun;59(6):1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23(10):1183–1195. doi: 10.1016/0042-6989(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J. A combined Golgi and autoradiographic study of 3H-glycine-accumulating cone bipolar cells in the cat retina. J Neurosci. 1987 Apr;7(4):1178–1188. doi: 10.1523/JNEUROSCI.07-04-01178.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson J. G., Troy J. B. Nature of the maintained discharge of Q, X, and Y retinal ganglion cells of the cat. J Opt Soc Am A. 1987 Dec;4(12):2301–2307. doi: 10.1364/josaa.4.002301. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]


