Abstract
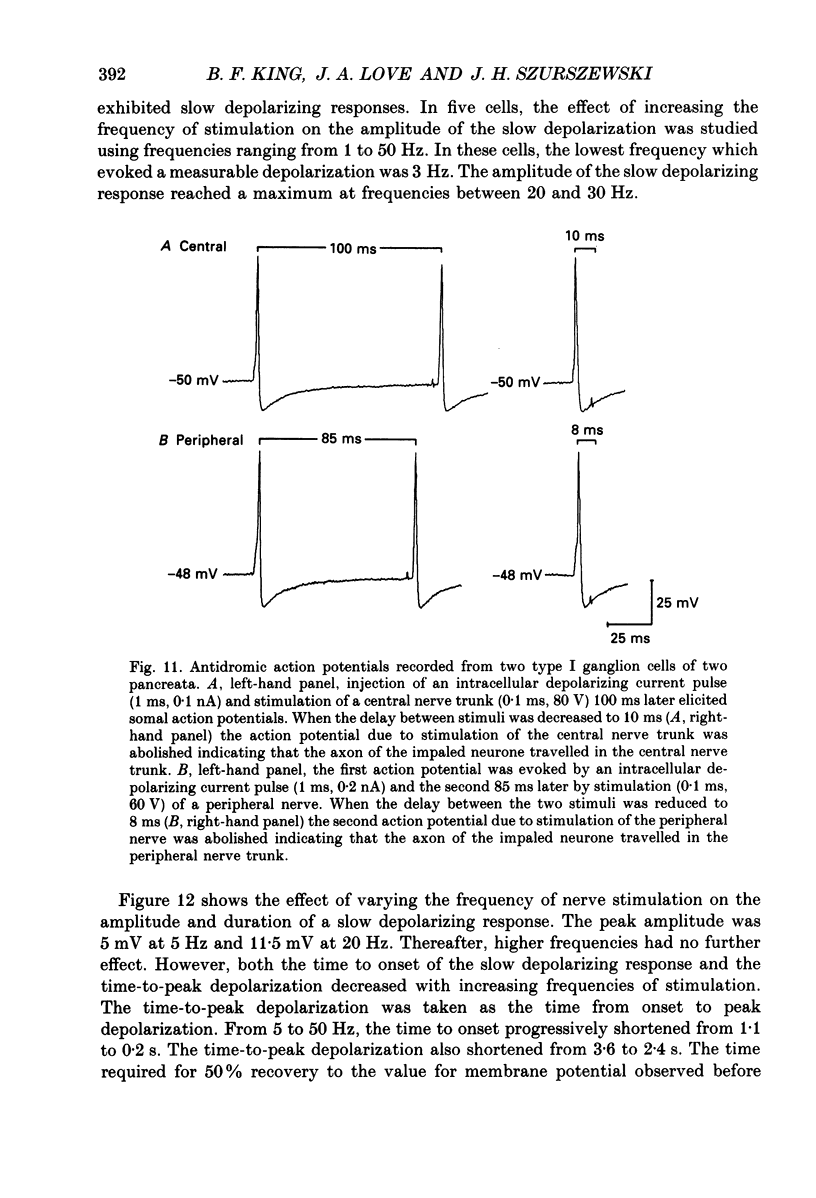
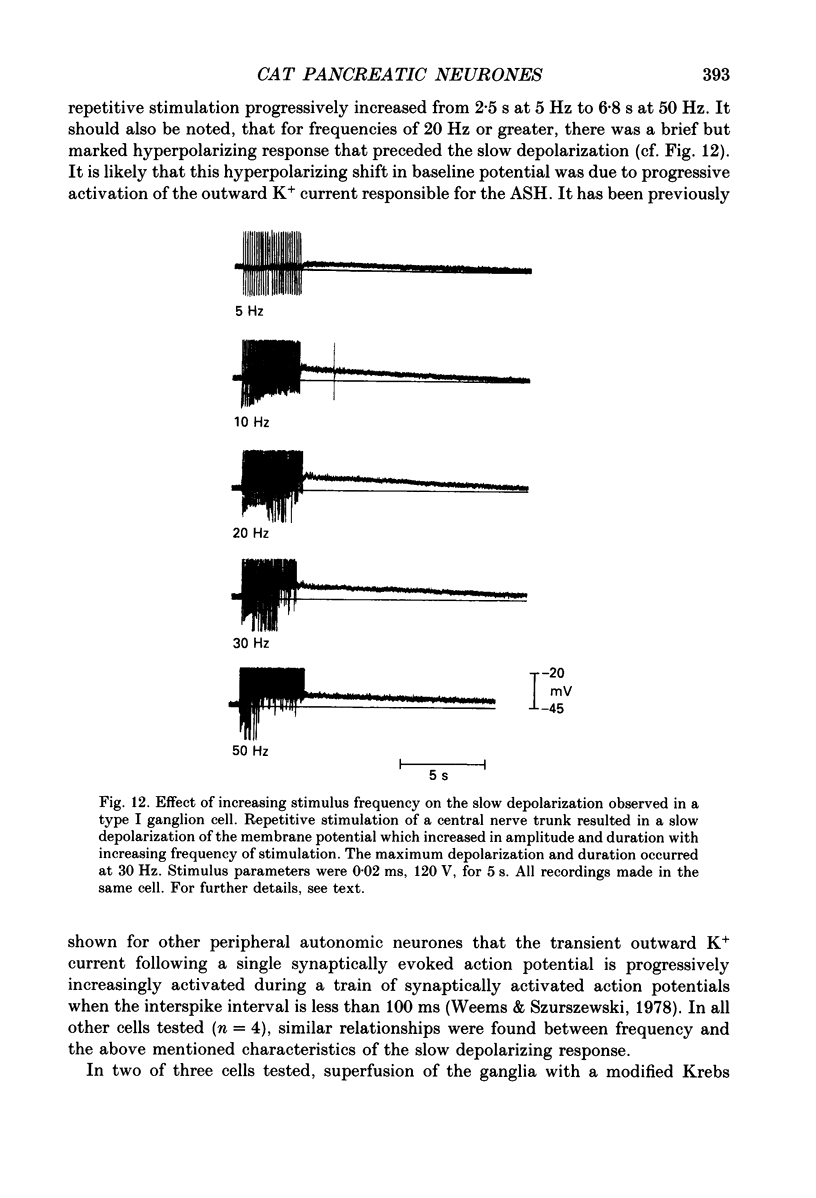
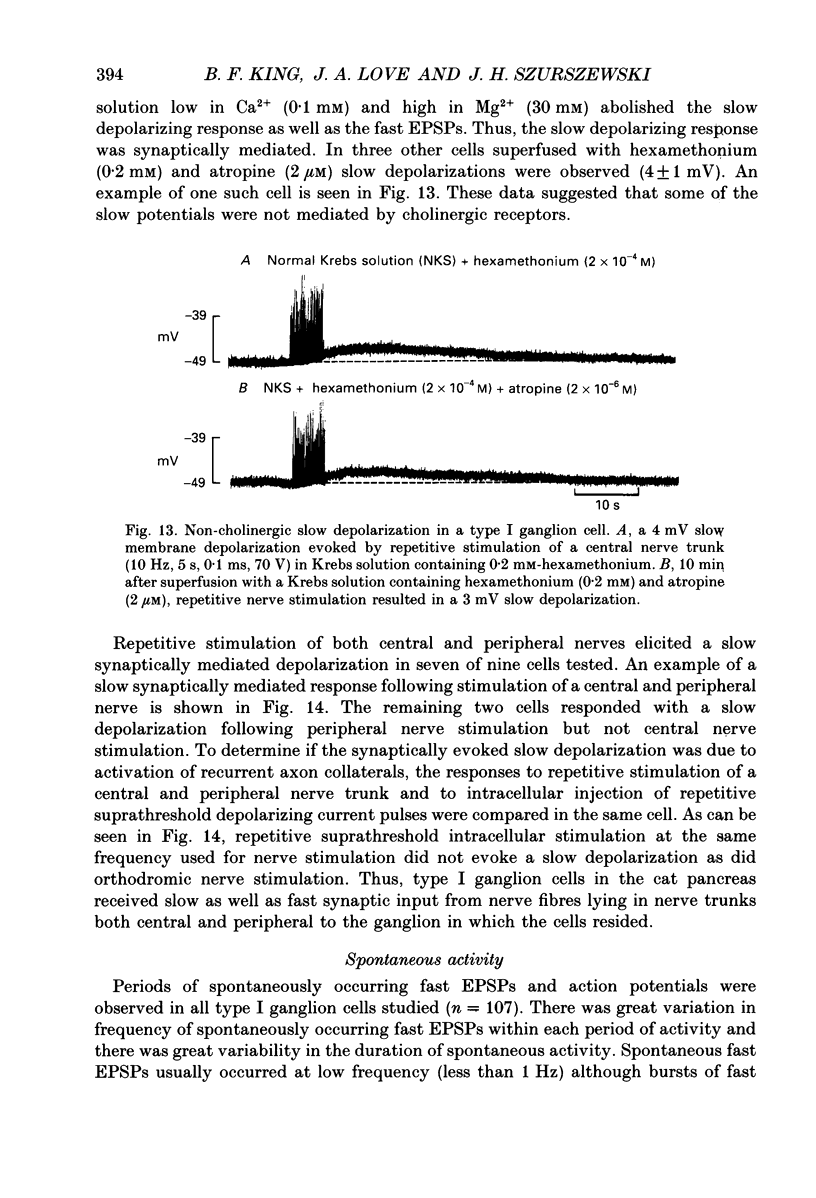
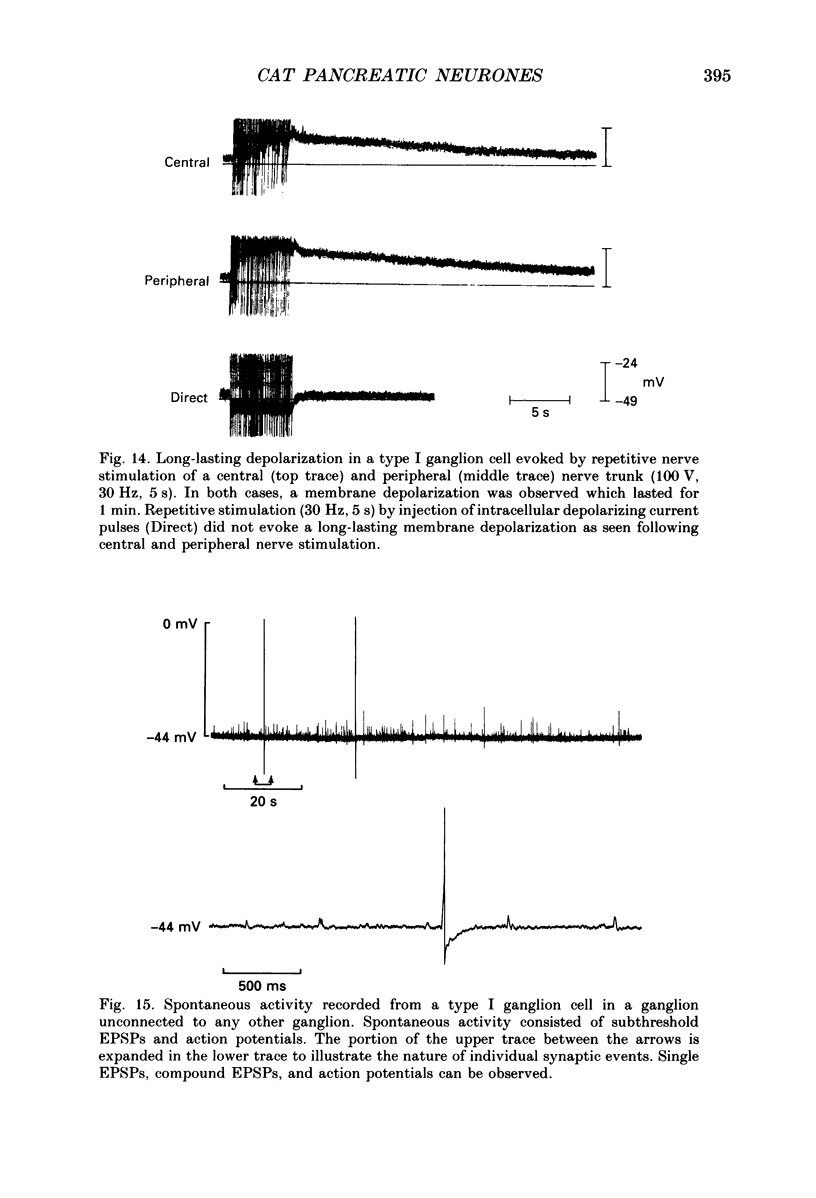
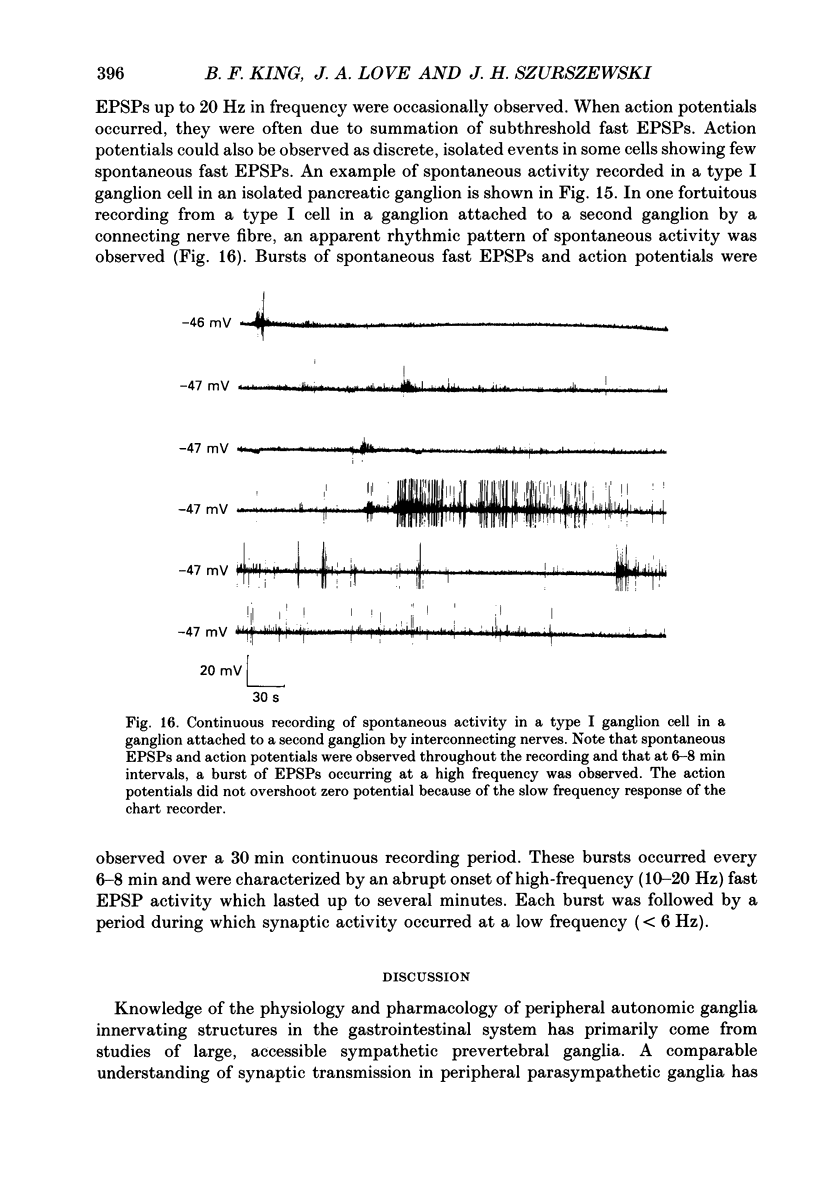
1. The anatomy, morphology, and electrophysiology of parasympathetic ganglia of cat pancreas were studied in vitro. 2. Pancreatic ganglia existed as an interconnected plexus of small ganglia (ten to fifty cells) lying in the interlobular connective tissue. Occasionally smaller ganglia (four to ten cells) were observed lying on or within nerve trunks. 3. Electron micrographs revealed the presence of neurones and satellite cells as well as unmyelinated axons and nerve terminals. Nerve terminals contained small clear vesicles and/or large, dense-cored vesicles. 4. Intracellular recording of electrical activity revealed the presence of two types of ganglion cells. Type I ganglion cells exhibited resting membrane potentials that ranged from -40 to -63 mV and input resistances that ranged from 8 to 168 M omega. They responded to intracellular depolarizing current with action potentials, and received synaptic inputs which when activated caused fast and slow depolarizing responses. Type I cells were considered to be ganglionic neurones. Type II ganglion cells had higher resting membrane potentials that ranged from -61 to -83 mV, lower input resistances that ranged from 5 to 83 M omega and were electrically unexcitable. Repetitive stimulation of preganglionic nerves evoked a slow depolarization that was frequency dependent. Type II cells were considered to be satellite cells. 5. Stimulation of nerve trunks both central and peripheral to the ganglia evoked multiple, subthreshold, fast EPSPs in all type I cells tested. Fast EPSPs were blocked by the nicotinic antagonist hexamethonium. 6. Antidromic potentials were also observed following stimulation of either central or peripheral nerve trunks but never both. 7. In type I cells repetitive stimulation of both central and peripheral nerve trunks resulted in a slow, synaptically mediated depolarization which persisted during superfusion with nicotinic and muscarinic receptor antagonists. 8. Periods of low-frequency, spontaneous fast EPSPs and action potentials were observed in all type I cells tested. 9. It was concluded that parasympathetic neurones in cat pancreatic ganglia receive convergent fast and slow synaptic inputs from central and possibly peripheral sources and may function in vivo as sites of integration. The occurrence of spontaneous synaptic potentials in pancreatic ganglia suggests the possibility of intrinsic neural control of pancreatic function.
Full text
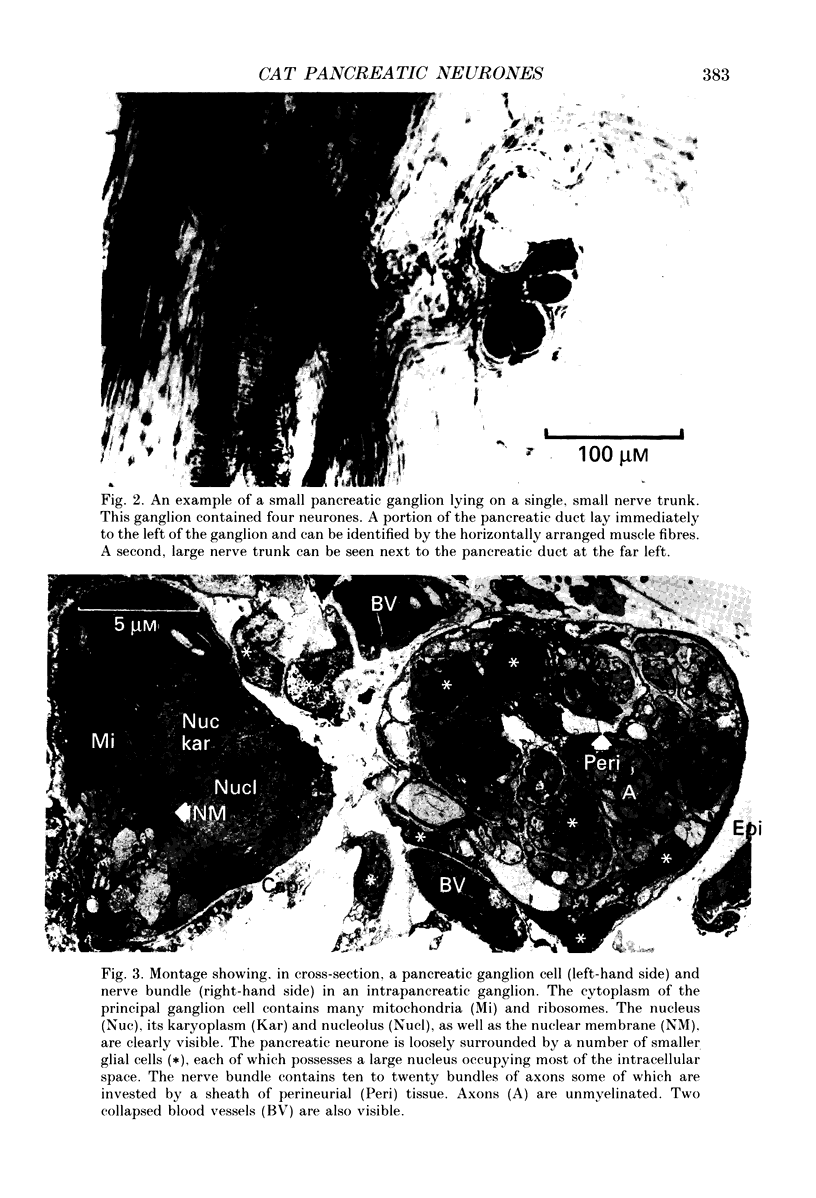
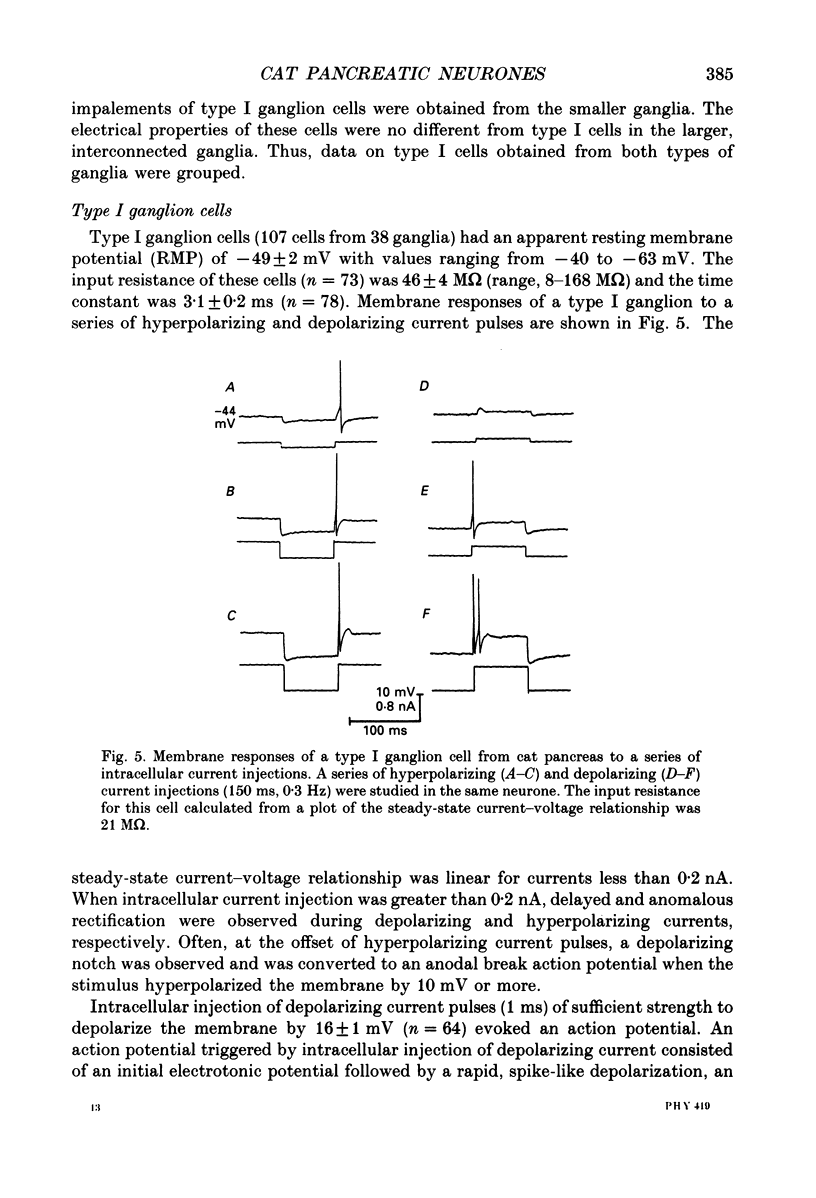
PDF




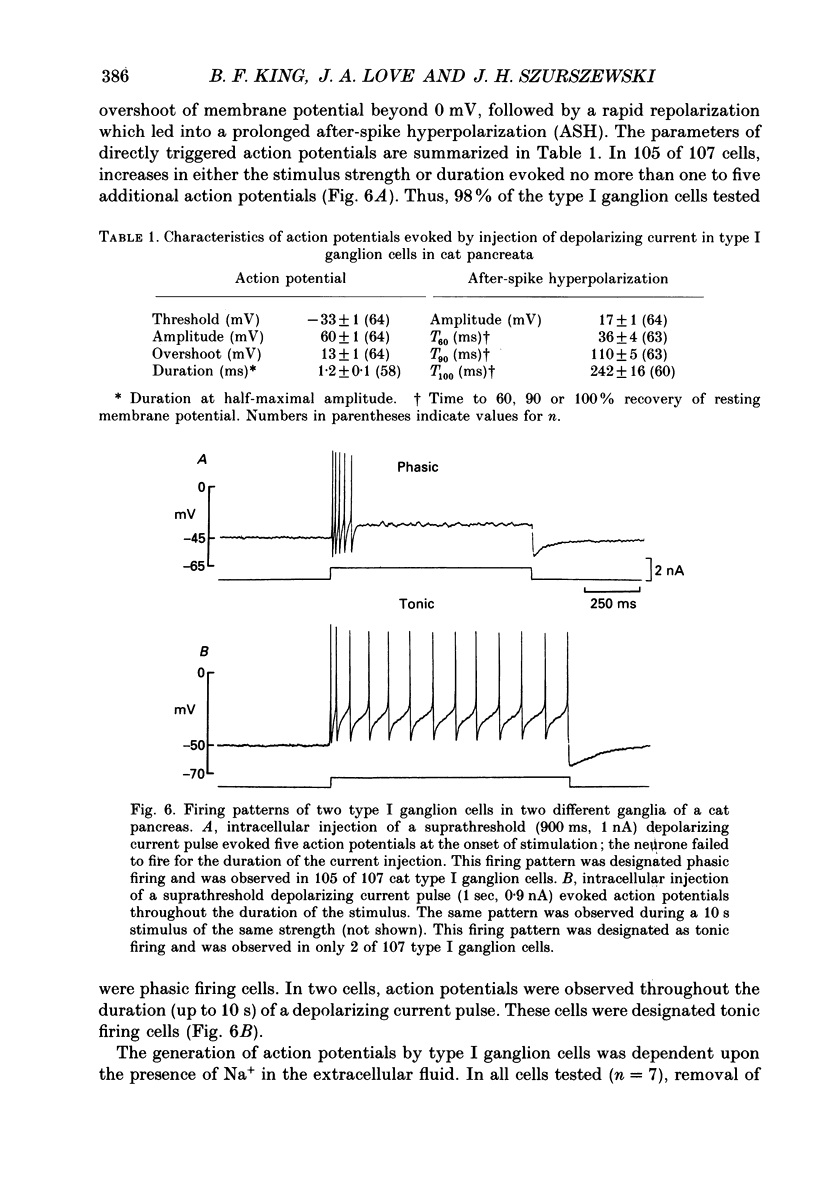
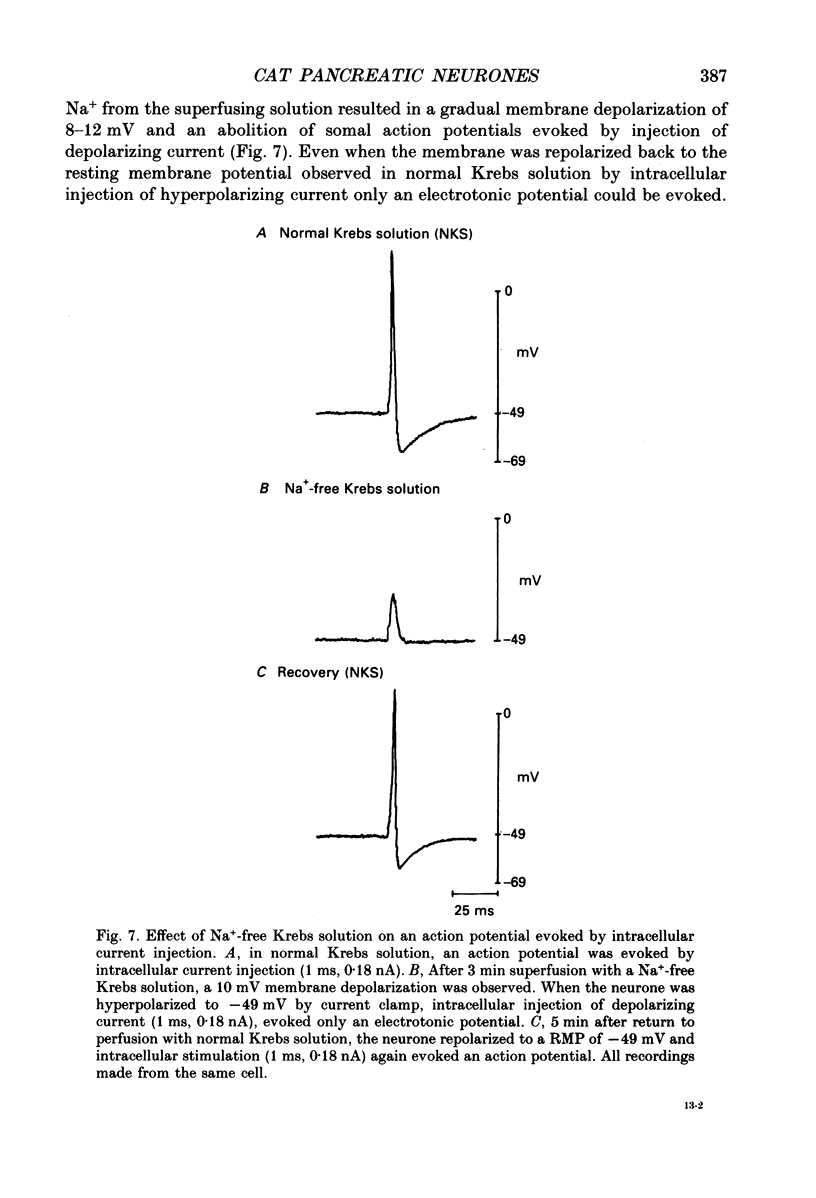
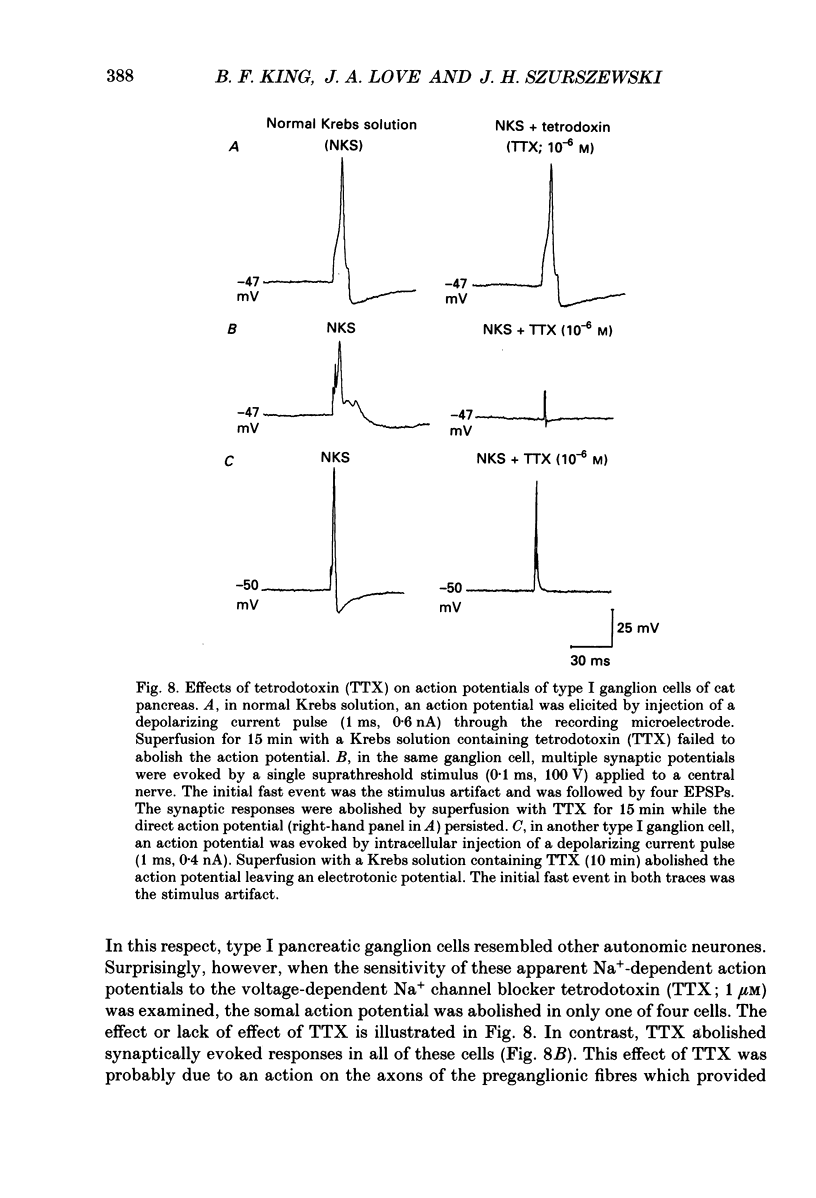

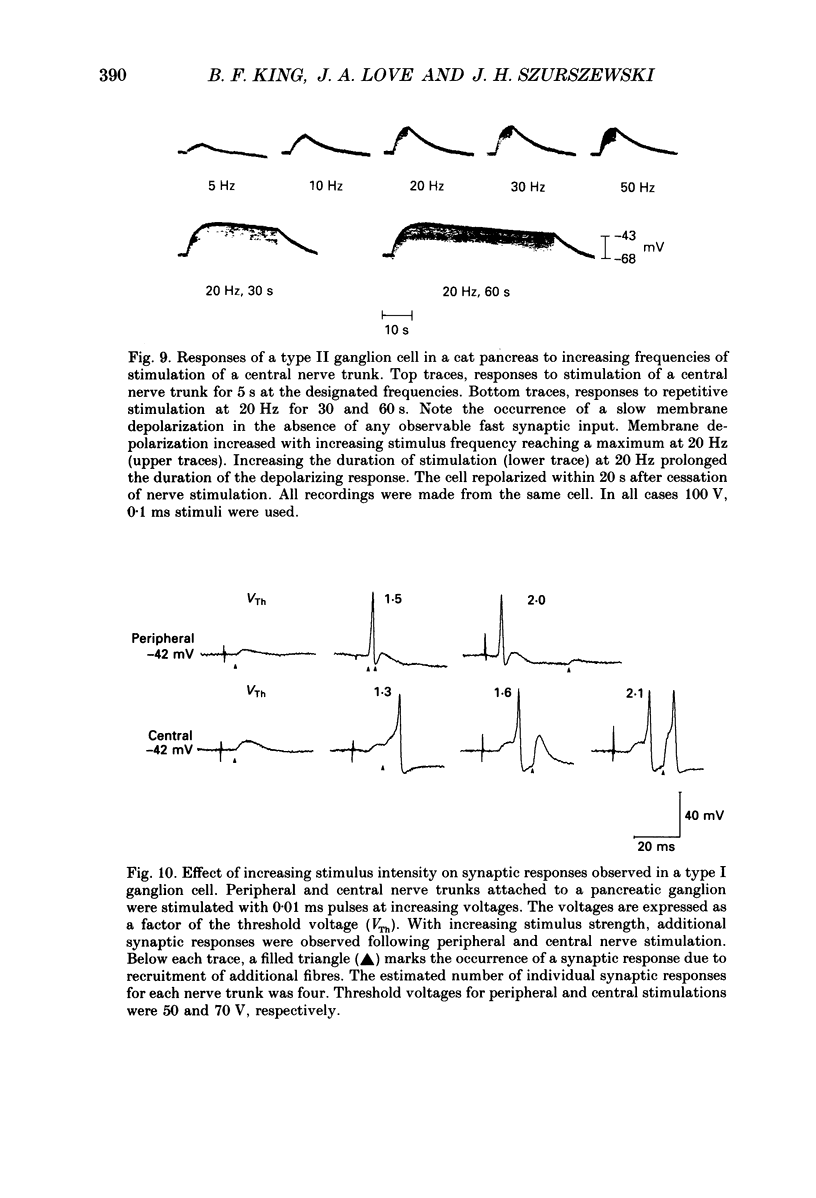








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Cegrell L., Ehinger B., Falck B. [Remarkable adrenergic nerves in the exocrine pancreas]. Z Zellforsch Mikrosk Anat. 1967;83(2):178–186. doi: 10.1007/BF00362399. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. M., DeGroat W. C. A study of facilitation in vesical parasympathetic ganglia of the cat using intracellular recording techniques. Brain Res. 1979 Jun 22;169(2):388–392. doi: 10.1016/0006-8993(79)91039-4. [DOI] [PubMed] [Google Scholar]
- COUPLAND R. E. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat. 1958 Jan;92(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Griffith W. H., Shinnick-Gallagher P. Cholinergic transmission in cat parasympathetic ganglia. J Physiol. 1982 Nov;332:473–486. doi: 10.1113/jphysiol.1982.sp014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980 Feb;43(2):343–354. doi: 10.1152/jn.1980.43.2.343. [DOI] [PubMed] [Google Scholar]
- King B. F., Szurszewski J. H. Intracellular recordings from vagally innervated intramural neurons in opossum stomach. Am J Physiol. 1984 Feb;246(2 Pt 1):G209–G212. doi: 10.1152/ajpgi.1984.246.2.G209. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Kumamoto E., Shinnick-Gallagher P. Postganglionic stimulation activates synaptic potentials in cat bladder parasympathetic neurons. Brain Res. 1987 Dec 1;435(1-2):403–407. doi: 10.1016/0006-8993(87)91634-9. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Innervation of the pancreas by substance P, enkephalin, vasoactive intestinal polypeptide and gastrin/CCK immunoractive nerves. J Histochem Cytochem. 1979 Sep;27(9):1283–1284. doi: 10.1177/27.9.479572. [DOI] [PubMed] [Google Scholar]
- Legg P. G. Fluorescence studies on neural structures and endocrine cells in the pancreas of the cat. Z Zellforsch Mikrosk Anat. 1968;88(4):487–495. doi: 10.1007/BF00571795. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J., Elde R., Said S. Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand. 1978 Dec;104(4):499–501. doi: 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981 Fall;2(4):471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- Sharkey K. A., Williams R. G. Extrinsic innervation of the rat pancreas: demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett. 1983 Dec 2;42(2):131–135. doi: 10.1016/0304-3940(83)90395-6. [DOI] [PubMed] [Google Scholar]
- Singh M., Webster P. D. Neurohormonal control of pancreatic secretion. A review. Gastroenterology. 1978 Feb;74(2 Pt 1):294–309. [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Modulation of insulin secretion by pancreatic ganglionic nicotinic receptors. Diabetes. 1986 Aug;35(8):849–854. doi: 10.2337/diab.35.8.849. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Perturbation of insulin oscillations by nerve blockade in the in vitro canine pancreas. Am J Physiol. 1985 May;248(5 Pt 1):E516–E521. doi: 10.1152/ajpendo.1985.248.5.E516. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol. 1985 May;248(5 Pt 1):E522–E530. doi: 10.1152/ajpendo.1985.248.5.E522. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E., Weir G. C. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. J Clin Invest. 1980 Apr;65(4):939–942. doi: 10.1172/JCI109750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kusano K. Hyperpolarizing potentials induced by Ca-mediated K-conductance increase in hamster submandibular ganglion cells. J Neurobiol. 1978 Sep;9(5):367–392. doi: 10.1002/neu.480090504. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sakada S. Synaptic transmission in the submandibular ganglion of the rat. Bull Tokyo Dent Coll. 1972 Aug;13(3):145–164. [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978 Mar;41(2):305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Woods S. C., Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev. 1974 Jul;54(3):596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]