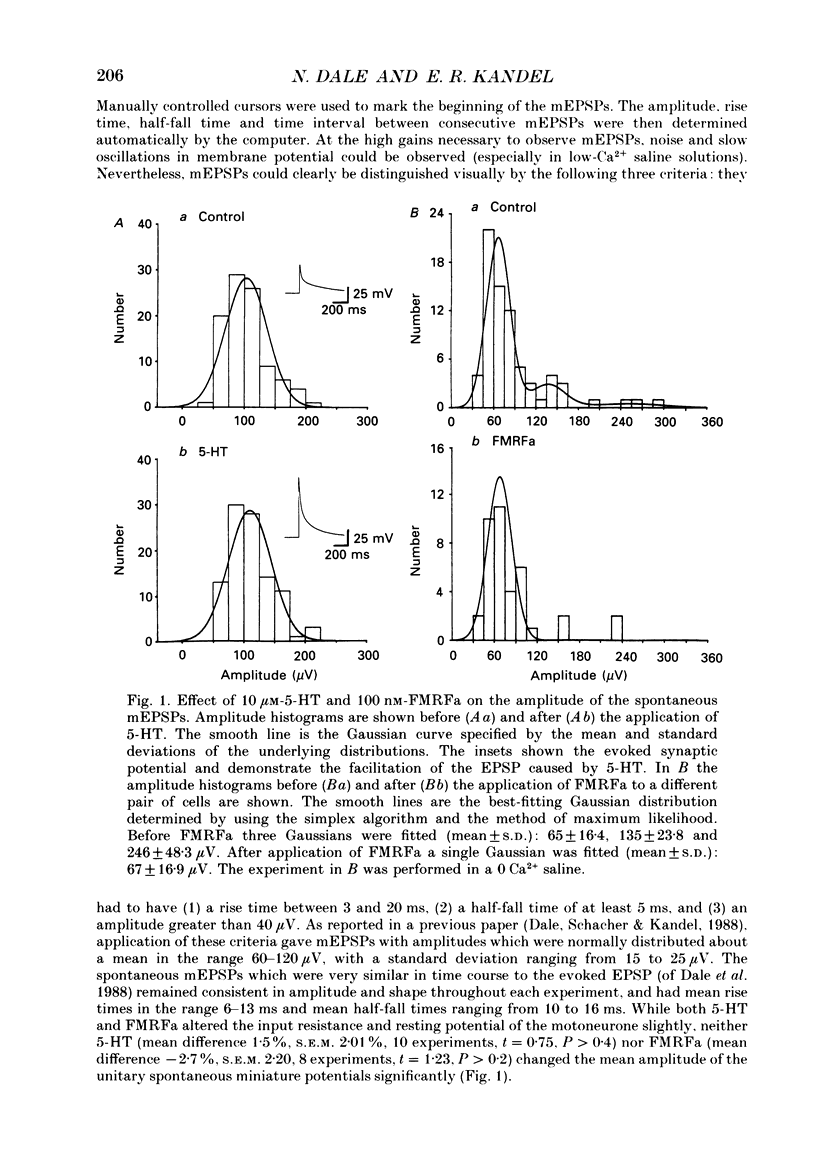
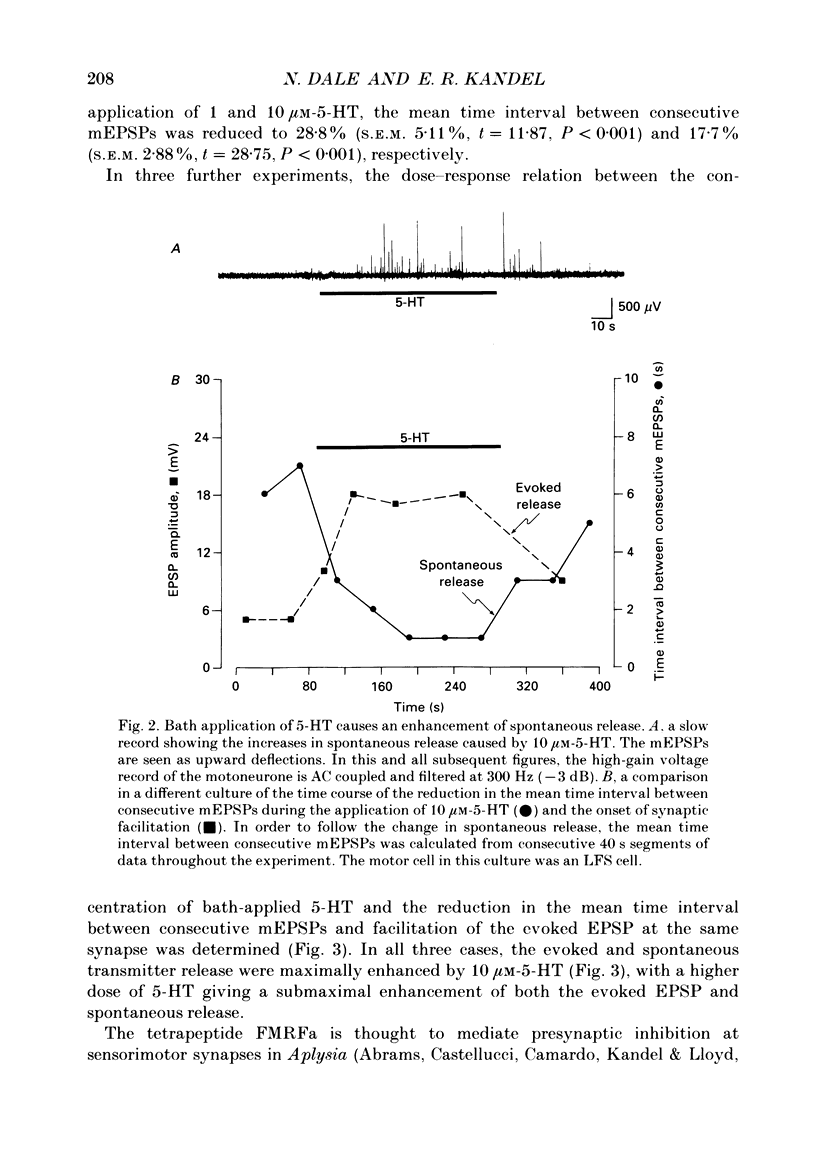
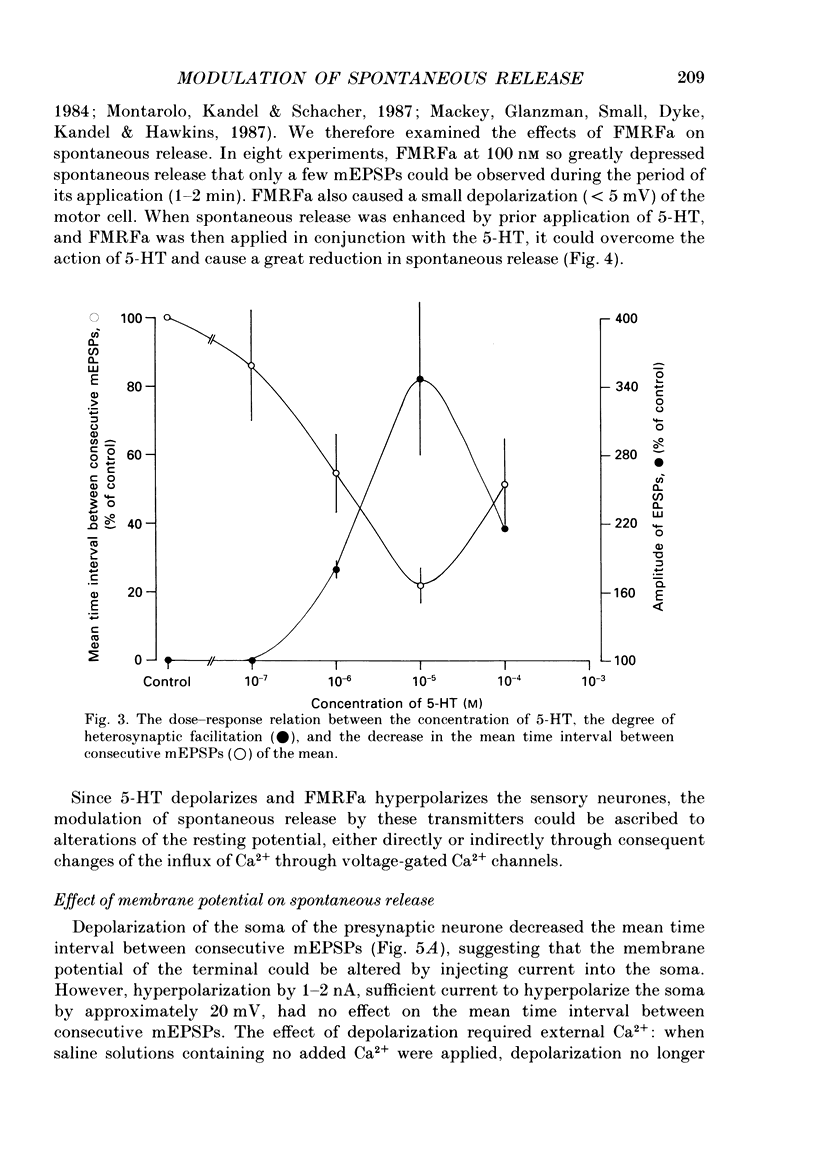
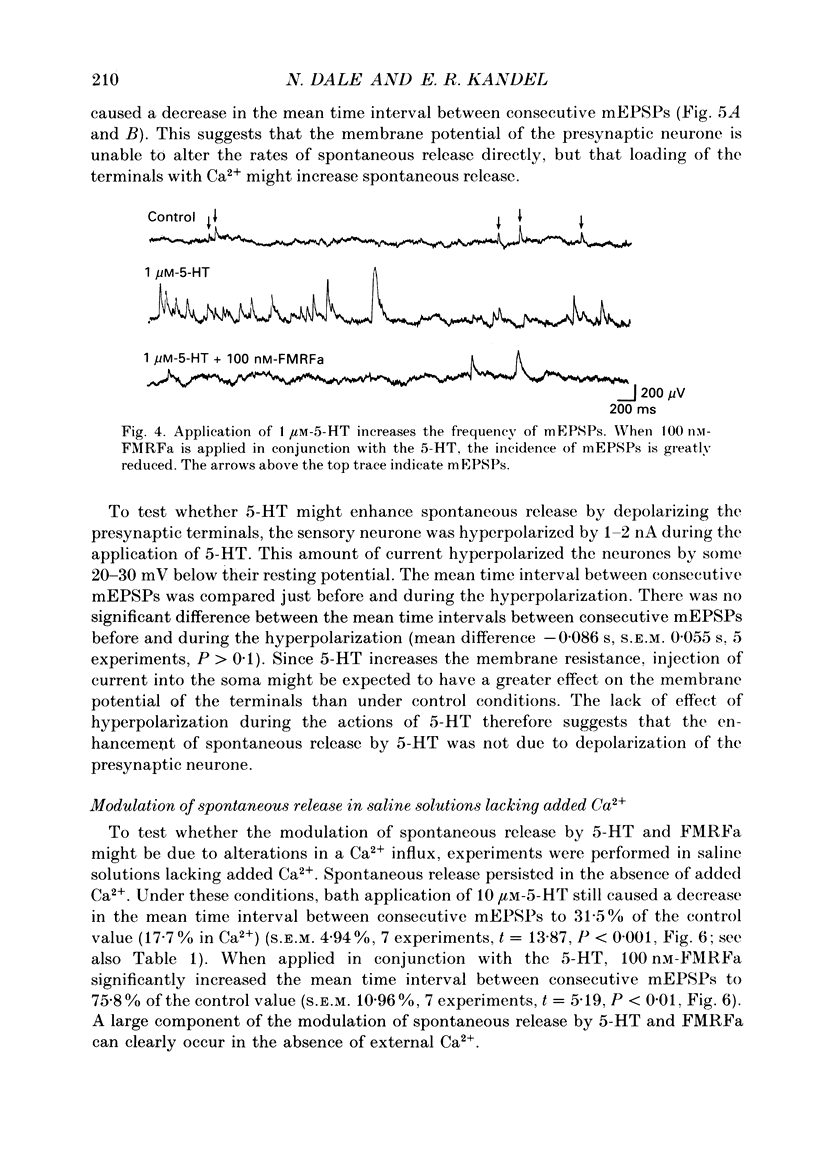
Abstract
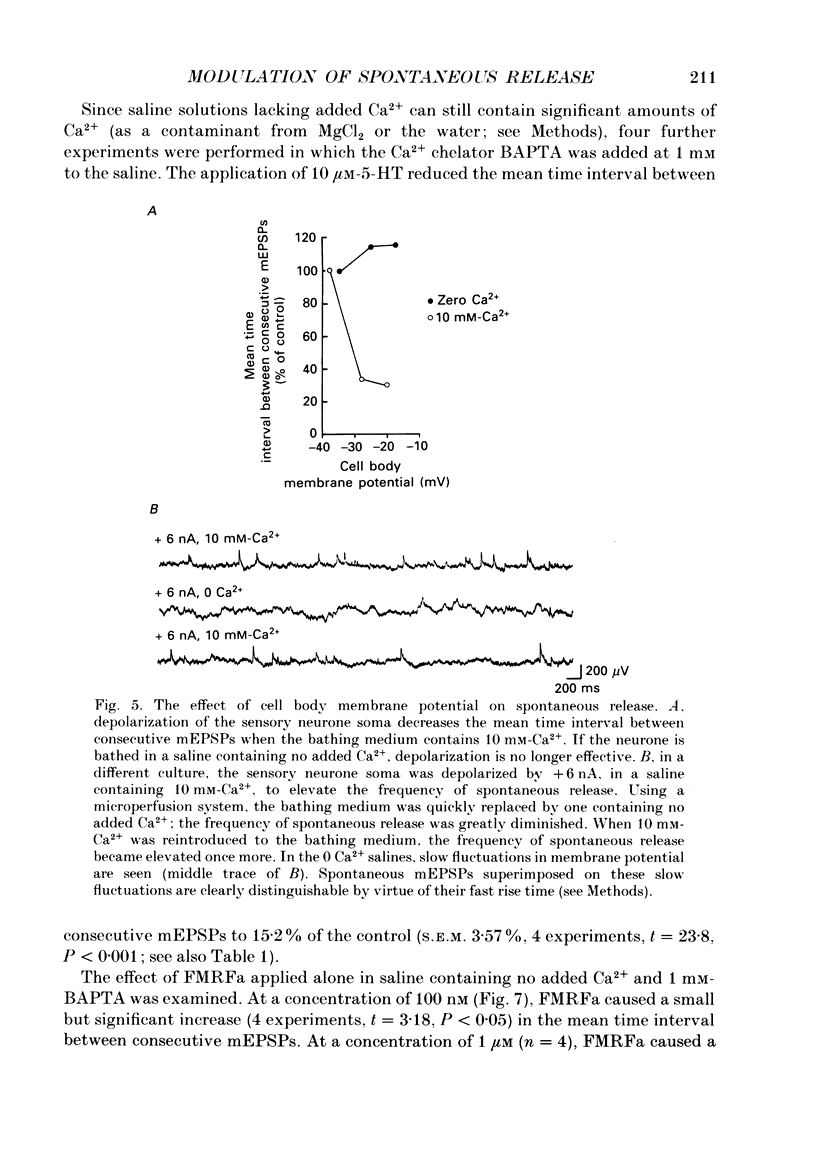
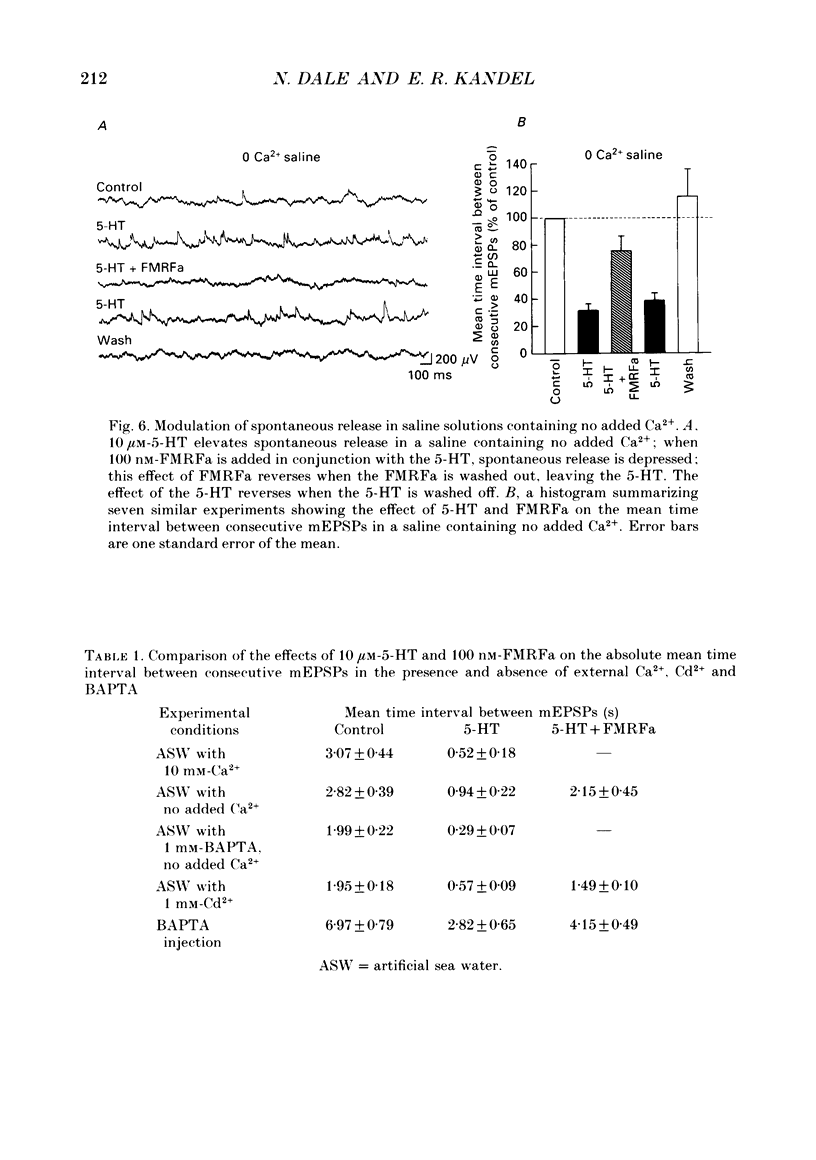
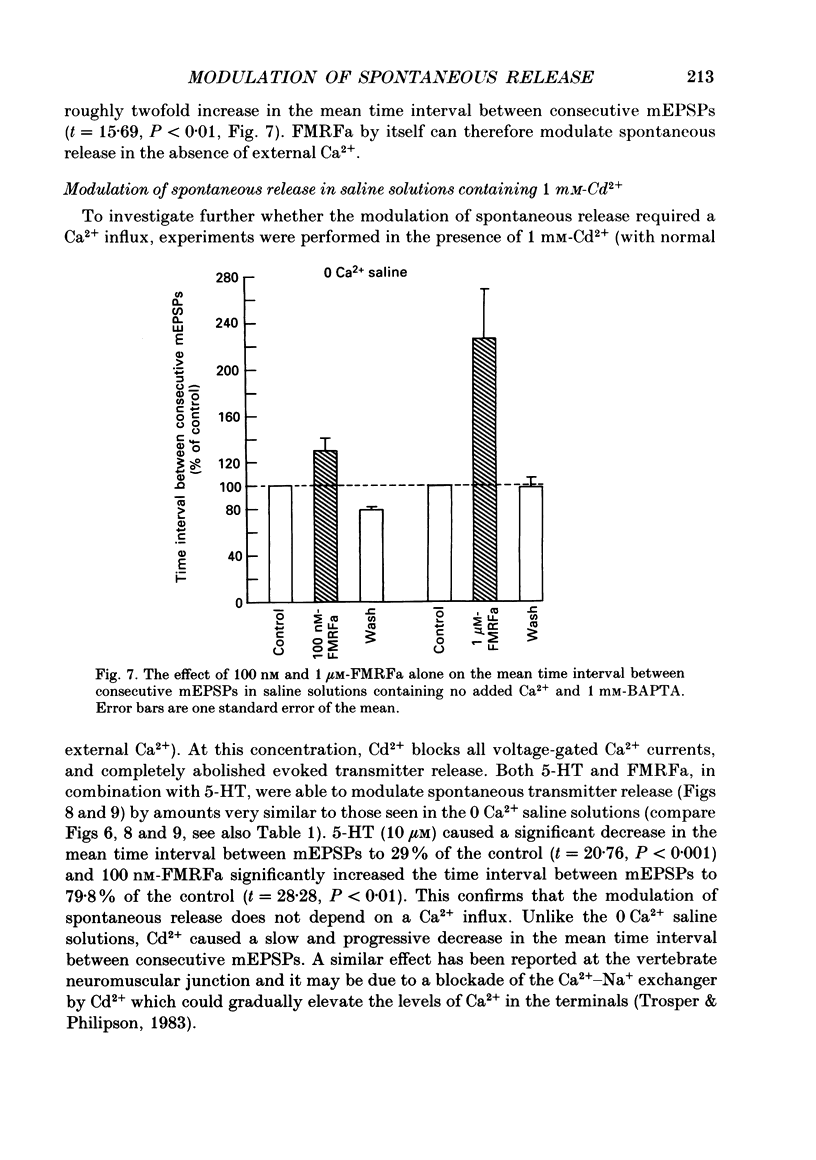
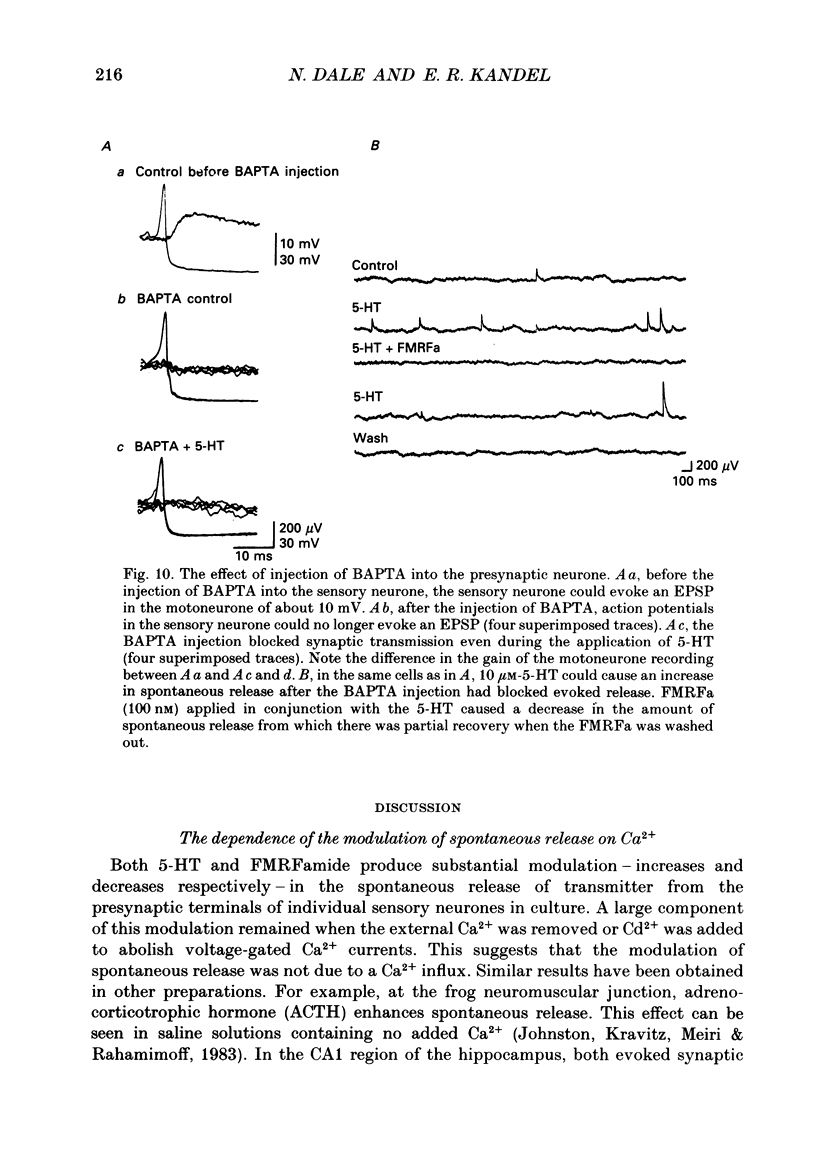
1. The monoamine transmitter 5-hydroxytryptamine (5-HT) and the peptide Phe-Met-Arg-Phe-amide (FMRFa), which appear to contribute to presynaptic facilitation and inhibition of the sensorimotor synapse in the abdominal ganglion of Aplysia, can modulate the frequency of spontaneous transmitter release at synapses formed between dissociated Aplysia sensory neurones and motoneurones in vitro. 2. 5-HT caused a decrease in the mean time interval between consecutive miniature EPSPs (mEPSPs), while FMRFa, applied either by itself or together with 5-HT, caused an increase in the mean time interval between consecutive mEPSPs. 3. Depolarization of the presynaptic neurone caused a decrease in the mean time interval between consecutive mEPSPs. This modulation required external Ca2+. 4. 5-HT and FMRFa were able to modulate spontaneous release when applied in saline solutions lacking Ca2+ and containing Ca2(+)-chelating agents, suggesting that the modulation of spontaneous release by 5-HT and FMRFa did not require a Ca2+ influx. Similarly, spontaneous release could still be modulated by 5-HT and FMRFa in saline solutions containing 1 mM-Cd2+, which blocked both the voltage-gated Ca2+ channels and the evoked transmitter release. 5. To prevent a rise in intracellular Ca2+, we buffered the concentration of Ca2+ in the presynaptic terminals by injecting into the sensory neurone the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA). The injection of BAPTA blocked evoked transmitter release, suggesting that it acted as an effective buffer of Ca2+ in the terminals. However, spontaneous release could still be observed and was still modulated by 5-HT and FMRFa. This suggests that the modulation of spontaneous release does not require an elevation of intracellular Ca2+. 6. We propose that 5-HT and FMRFa can modulate the rate of spontaneous release directly by mechanisms that do not require changes in the intracellular concentration of Ca2+. These mechanisms might contribute an additional component to the presynaptic inhibition and facilitation of evoked transmitter release.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Kandel E. R., Siegelbaum S. A. Neuronal inhibition by the peptide FMRFamide involves opening of S K+ channels. Nature. 1987 Jan 8;325(7000):153–156. doi: 10.1038/325153a0. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Schacher S., Kandel E. R., Siegelbaum S. A. The growth cones of Aplysia sensory neurons: Modulation by serotonin of action potential duration and single potassium channel currents. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7094–7098. doi: 10.1073/pnas.83.18.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. B., Klein M., Smith S. J., Kandel E. R. Serotonin increases intracellular Ca2+ transients in voltage-clamped sensory neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7642–7646. doi: 10.1073/pnas.81.23.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V., Eckert R., Erxleben C. Suppression of calcium current by an endogenous neuropeptide in neurones of Aplysia californica. J Physiol. 1987 Jul;388:565–595. doi: 10.1113/jphysiol.1987.sp016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Schacher S., Kandel E. R. Long-term facilitation in Aplysia involves increase in transmitter release. Science. 1988 Jan 15;239(4837):282–285. doi: 10.1126/science.2892269. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Greengard P. Synapsin I: a synaptic vesicle-associated neuronal phosphoprotein. Biochem Pharmacol. 1986 Dec 15;35(24):4349–4357. doi: 10.1016/0006-2952(86)90747-1. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gingrich K. J., Byrne J. H. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985 Mar;53(3):652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- Higashida H. Acetylcholine release by bradykinin, inositol 1,4,5-trisphosphate and phorbol dibutyrate in rodent neuroblastoma cells. J Physiol. 1988 Mar;397:209–222. doi: 10.1113/jphysiol.1988.sp016996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8794–8798. doi: 10.1073/pnas.83.22.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Kravitz E. A., Meiri H., Rahamimoff R. Adrenocorticotropic hormone causes long-lasting potentiation of transmitter release from frog motor nerve terminals. Science. 1983 Jun 3;220(4601):1071–1072. doi: 10.1126/science.6133353. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Bailey C. H., Chen M., Kandel E. R. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. II. Presynaptic conductance changes caused by histamine. J Neurophysiol. 1986 Jan;55(1):131–146. doi: 10.1152/jn.1986.55.1.131. [DOI] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Kandel E. R. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. I. Physiological mechanisms. J Neurophysiol. 1986 Jan;55(1):113–130. doi: 10.1152/jn.1986.55.1.113. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A., Rahamimoff R. A study of tetanic and post-tetanic potentiation of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1980 Dec;309:247–273. doi: 10.1113/jphysiol.1980.sp013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S. L., Glanzman D. L., Small S. A., Dyke A. M., Kandel E. R., Hawkins R. D. Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8730–8734. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Ayoub G. S., Nicoll R. A. Phorbol esters enhance transmitter release in rat hippocampal slices. Brain Res. 1987 Feb 10;403(1):198–203. doi: 10.1016/0006-8993(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Montarolo P. G., Kandel E. R., Schacher S. Long-term heterosynaptic inhibition in Aplysia. Nature. 1988 May 12;333(6169):171–174. doi: 10.1038/333171a0. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Rayport S. G., Schacher S. Synaptic plasticity in vitro: cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci. 1986 Mar;6(3):759–763. doi: 10.1523/JNEUROSCI.06-03-00759.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983 Dec;3(12):2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987 Dec 1;435(1-2):387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Trosper T. L., Philipson K. D. Effects of divalent and trivalent cations on Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983 May 26;731(1):63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Haydon P. G. Membrane potential has no direct role in evoking neurotransmitter release. Nature. 1988 Sep 22;335(6188):360–362. doi: 10.1038/335360a0. [DOI] [PubMed] [Google Scholar]


