Abstract
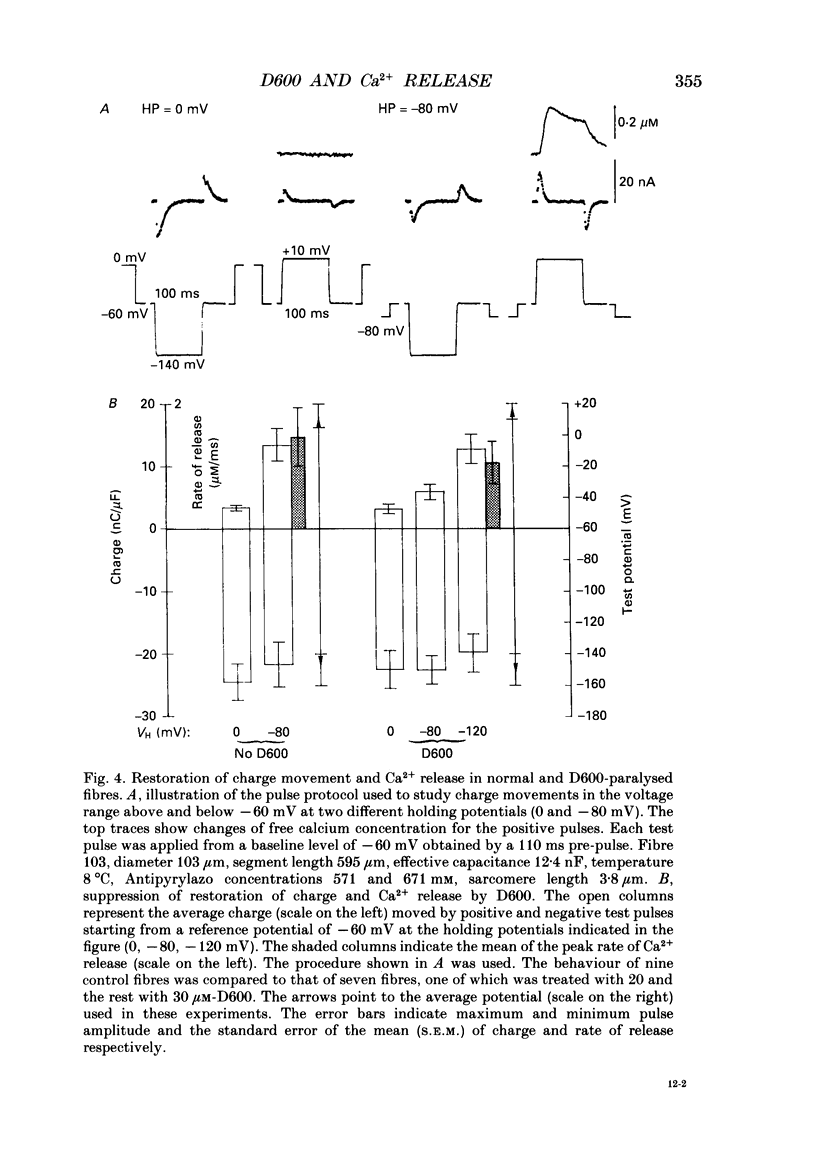
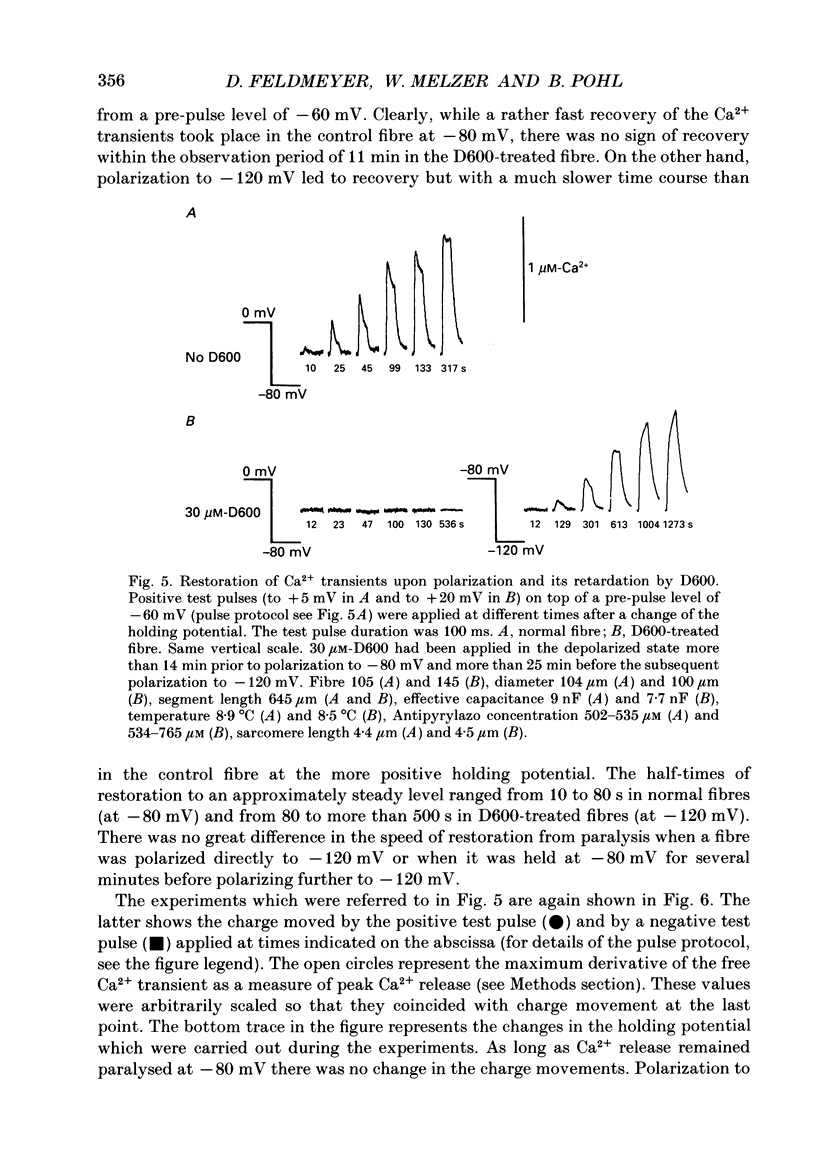
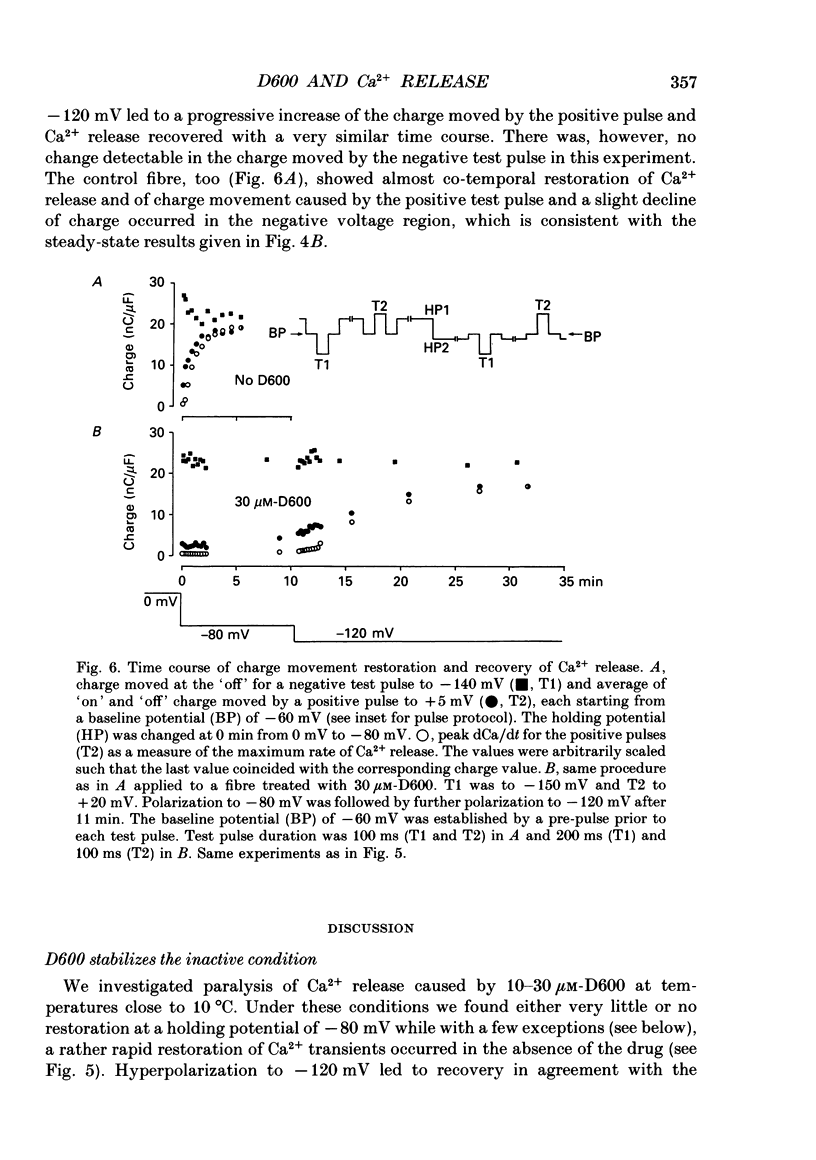
1. Intramembrane charge movements and changes in intracellular Ca2+ concentration were studied in voltage clamp experiments on cut twitch muscle fibres of the frog. The restoration from inactivation caused by steady depolarization and its modification by the phenylalkylamine Ca2+ channel antagonist gallopamil (D600, 10-30 microM) were investigated. 2. D600 prevented the restoration from inactivation of Ca2+ release which normally occurred at -80 mV. In D600 Ca2+ release recovered from inactivation at -120 mV. 3. D600 did not alter the characteristics of intramembrane charge movements in the depolarized fibre (charge 2) but the increase in the amount of mobile charge in the test voltage range above -60 mV, which normally occurs after changing the holding potential to -80 mV, was suppressed. The charge movement characteristics of D600-paralysed fibres, which were held at -80 mV, equalled those of normal depolarized and inactivated fibres. 4. Control records for the charge movement analysis were always obtained by voltage steps above 0 mV. Using the 'conventional' control in the potential range between -80 and -160 mV led to an underestimation and a kinetic deformation of charge movements in D600-treated fibres, which was due to various amounts of nonlinear charge in the control. 5. Like the restoration of Ca2+ release at -80 mV in normal fibres the recovery from paralysis at -120 mV in D600-treated fibres was accompanied by a significant increase in mobile charge in the potential range positive of -60 mV. Both Ca2+ release and charge movement at test potentials above -60 mV recovered with almost identical time course. 6. Restoration of Ca2+ release at a holding potential of -80 mV in normal fibres or at -120 mV in D600-treated fibres could not be clearly correlated to charge movement changes in the voltage range negative of -60 mV (charge 2). 7. Our results are consistent with a voltage-dependent inhibitory effect of D600 on the charge displacement that controls Ca2+ release from the sarcoplasmic reticulum but provide little evidence for a conversion of charge 2 into the charge that is involved in the control of Ca2+ release.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Rakowski R. F. Charge movement and mechanical repriming in skeletal muscle. J Physiol. 1976 Jan;254(2):361–388. doi: 10.1113/jphysiol.1976.sp011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. S. Dual roles for DHP receptors in excitation--contraction coupling? Nature. 1987 Jul 23;328(6128):297–297. doi: 10.1038/328297a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol. 1988 Apr;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Rios E. Intramembrane charge movement in frog skeletal muscle fibres. Properties of charge 2. J Physiol. 1987 Jun;387:489–517. doi: 10.1113/jphysiol.1987.sp016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Bolaños P. Effects of D-600 on intramembrane charge movement of polarized and depolarized frog muscle fibers. J Gen Physiol. 1989 Jul;94(1):43–64. doi: 10.1085/jgp.94.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Lüttgau H. C. The effect of the phenylalkylamine D888 (devapamil) on force and Ca2+ current in isolated frog skeletal muscle fibres. J Physiol. 1989 Jun;413:521–541. doi: 10.1113/jphysiol.1989.sp017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M. D., Best P. M. Block of contracture in skinned frog skeletal muscle fibers by calcium antagonists. J Gen Physiol. 1989 Mar;93(3):429–449. doi: 10.1085/jgp.93.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Striessnig J., Schober M., Glossmann H. (-)-[3H]Desmethoxyverapamil, a novel Ca2+ channel probe. Binding characteristics and target size analysis of its receptor in skeletal muscle. FEBS Lett. 1984 Oct 29;176(2):371–377. doi: 10.1016/0014-5793(84)81199-0. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Hui C. S., Milton R. L., Eisenberg R. S. Charge movement in skeletal muscle fibers paralyzed by the calcium-entry blocker D600. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2582–2585. doi: 10.1073/pnas.81.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Milton R. L. Suppression of charge movement in frog skeletal muscle by D600. J Muscle Res Cell Motil. 1987 Jun;8(3):195–208. doi: 10.1007/BF01574588. [DOI] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Asymmetric charge movement in polarized and depolarized muscle fibres of the rabbit. J Physiol. 1987 Feb;383:349–367. doi: 10.1113/jphysiol.1987.sp016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J. 1987 Jun;51(6):849–863. doi: 10.1016/S0006-3495(87)83413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M. F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol. 1986 Mar;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Siebler M., Schmidt H. D600 prolongs inactivation of the contractile system in frog twitch fibres. Pflugers Arch. 1987 Sep;410(1-2):75–82. doi: 10.1007/BF00581899. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]


