Abstract
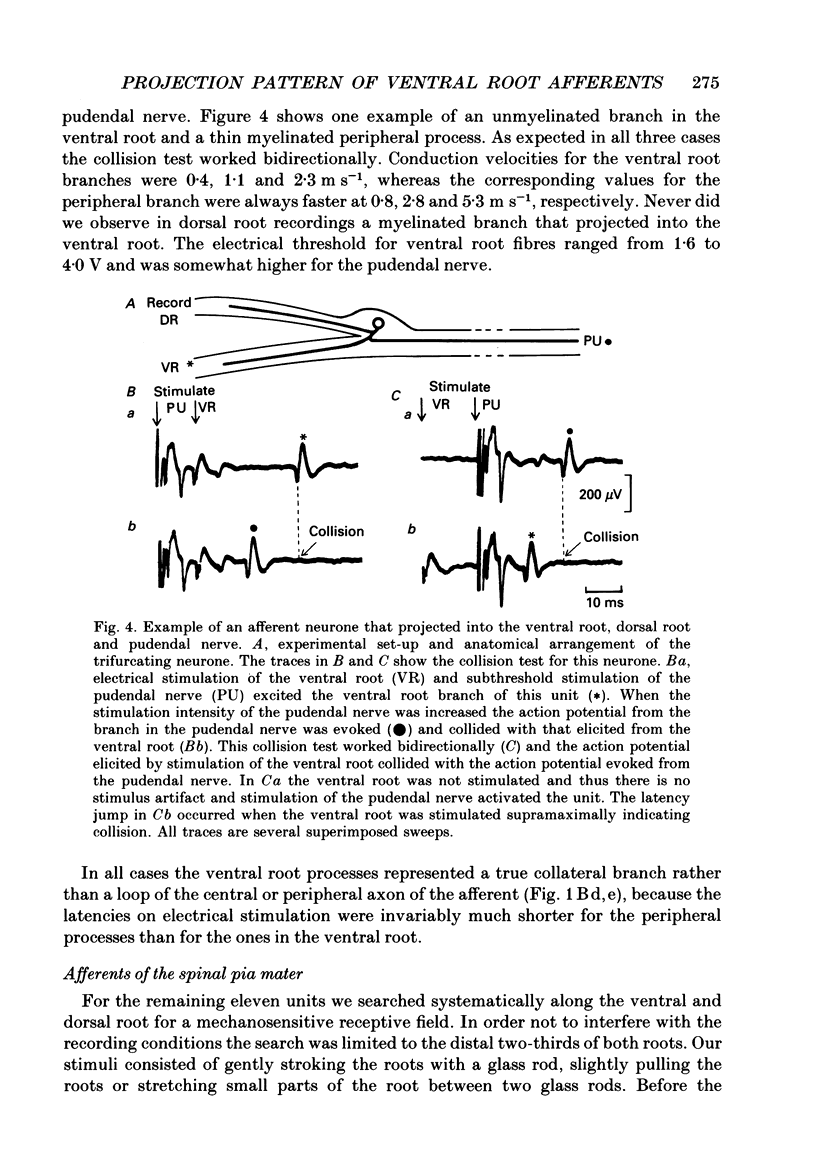
1. The ventral roots of the spinal cord contain a large number of unmyelinated primary afferent neurones. There is some controversy, however, about the function of these fibres and the route of their central projection. Here we have used electrophysiological techniques to quantify the central projection patterns of these neurones in the segment S2 of adult chloralose-anaesthesized cats. 2. A total of 1185 single unmyelinated units were recorded in small filaments isolated from intact and de-efferented ventral roots or intact dorsal roots of the segment S2 in nineteen cats. The projection patterns of these neurones were tested using supramaximal electrical stimulation of the pelvic and pudendal nerve (the main tributaries of the spinal nerve of this segment) and of the segmental companion root (dorsal or ventral as appropriate). 3. The principal finding of this study is that 85% of unmyelinated afferent axons in the ventral root are direct and exclusive projections. They constitute a separate class of afferents which is only capable of transmitting information from the periphery via the ventral roots. However, the proportion of these fibres that enter the central nervous system is unknown and it seems likely that some of them peter out as they approach the spinal cord and end blindly. The functional role of such afferents remains obscure. 4. For the remaining 15% of unmyelinated ventral root afferents, a projection into the segmental dorsal root was found. The majority of those fibres (about two-thirds) are primary afferent neurones innervating the pia mater. Some of these units had a small spot-like receptive field and responded to mechanical stimuli such as pressure and stretch of the root. They did not have axon projections in a peripheral nerve. 5. A few (5%) unmyelinated ventral root fibres are collateral branches of normal primary afferents projecting through the dorsal root. These trifurcating neurones are a small population which make up only some 0.5% of all dorsal root ganglion cells. The functional significance of this population too is unknown. 6. For none of the fibres that projected into both dorsal and ventral root was there positive evidence for the existence of looping axons that merely make a detour into one of the roots. Although the existence of loops cannot completely be excluded, our evidence suggests that they can constitute at most 5% of the unmyelinated ventral root afferents.
Full text
PDF


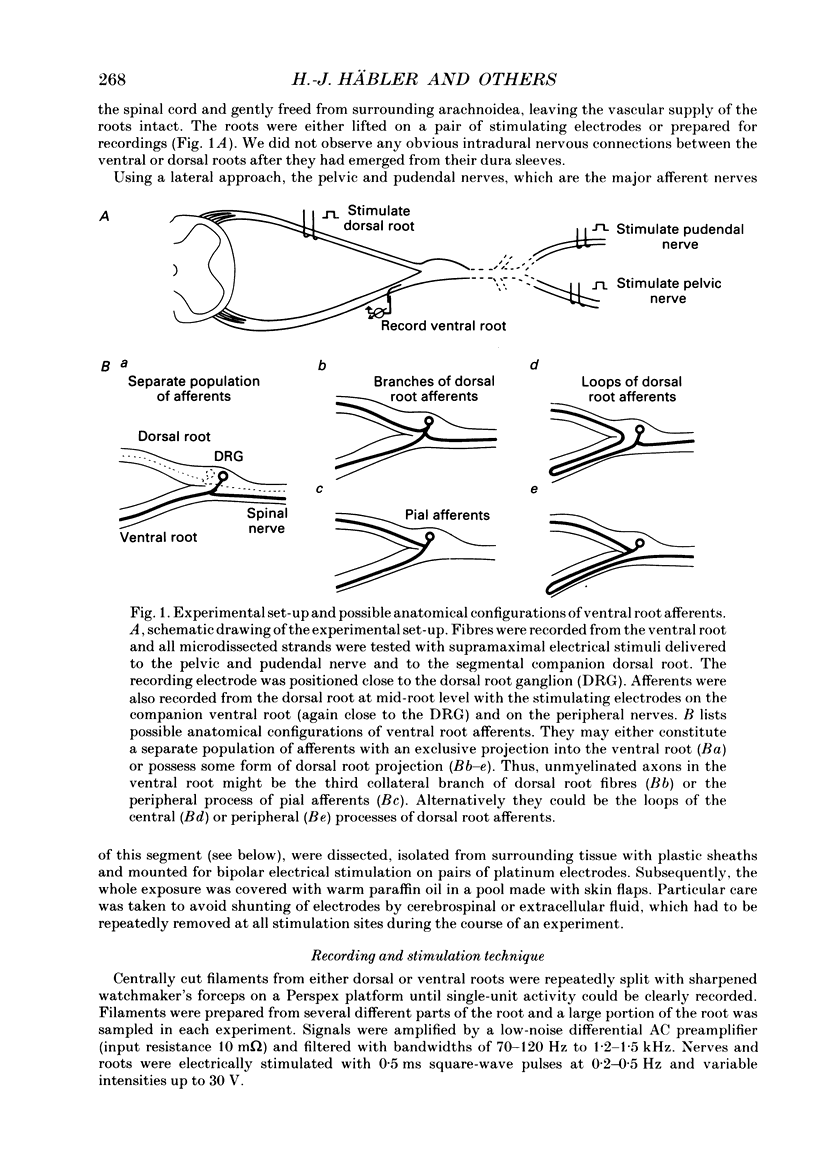



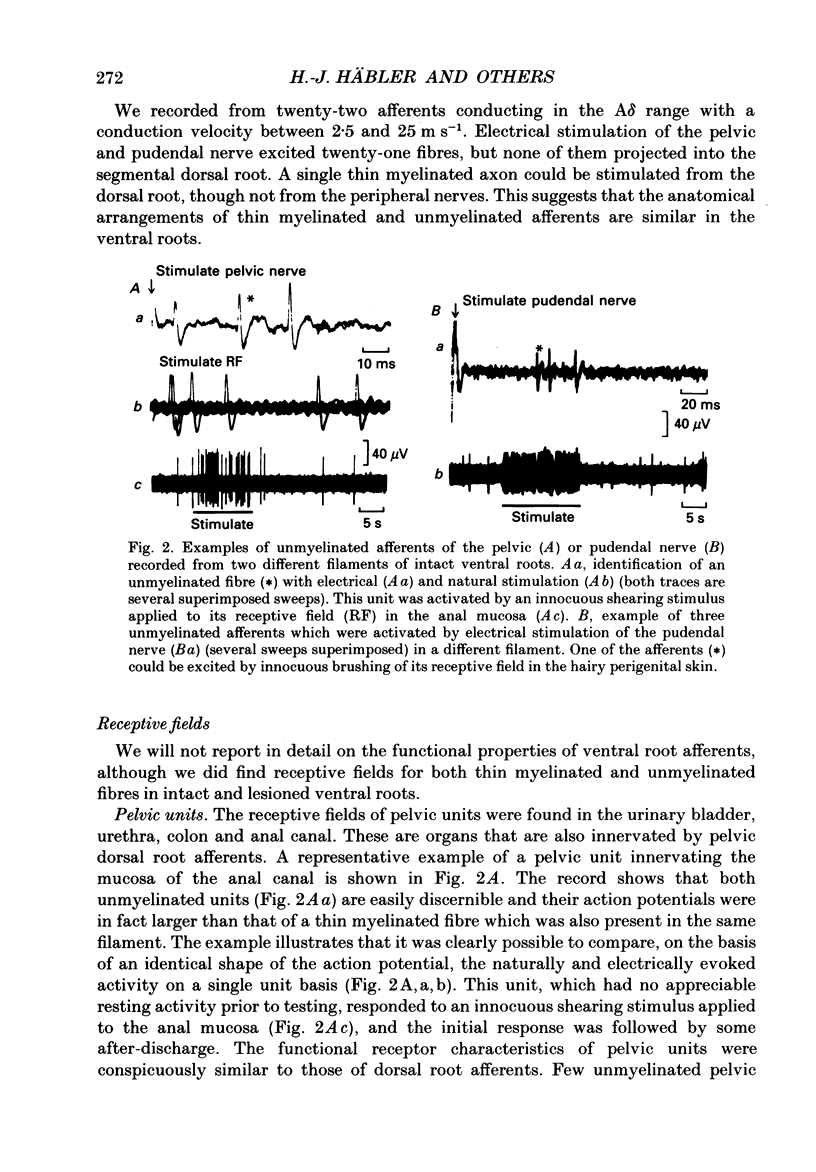

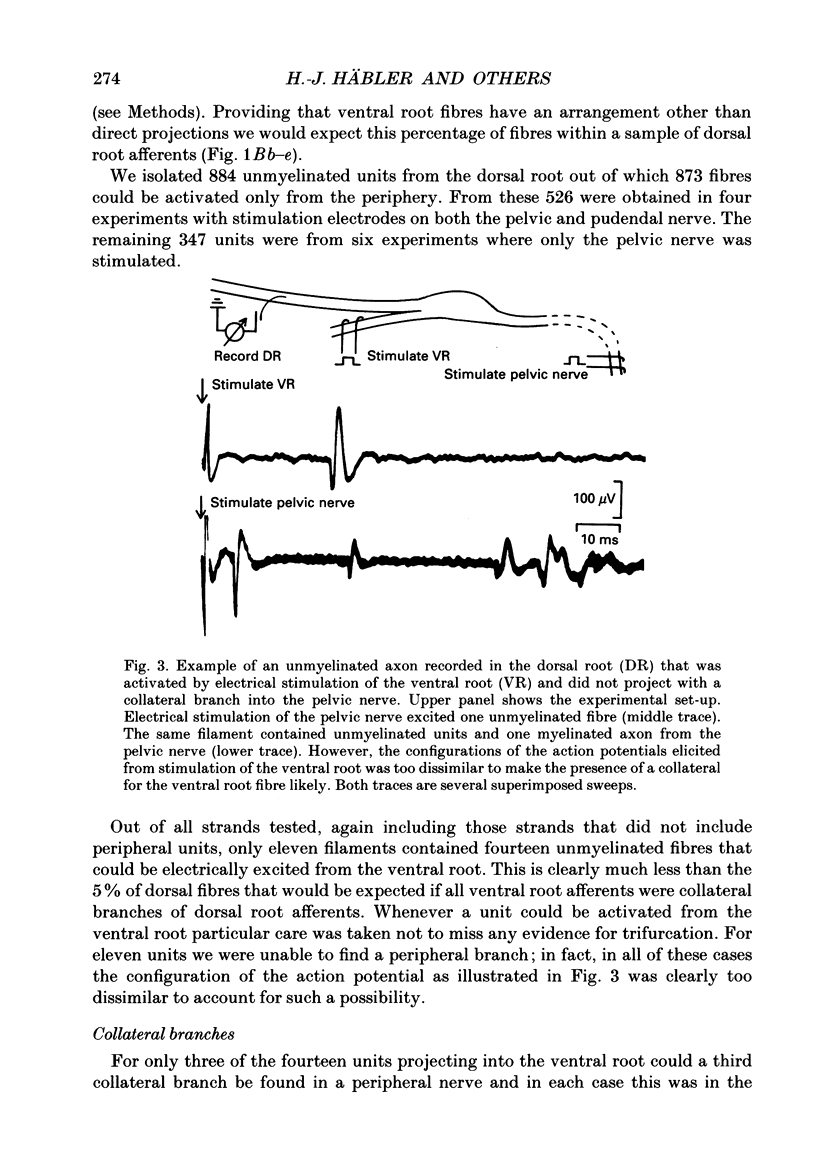
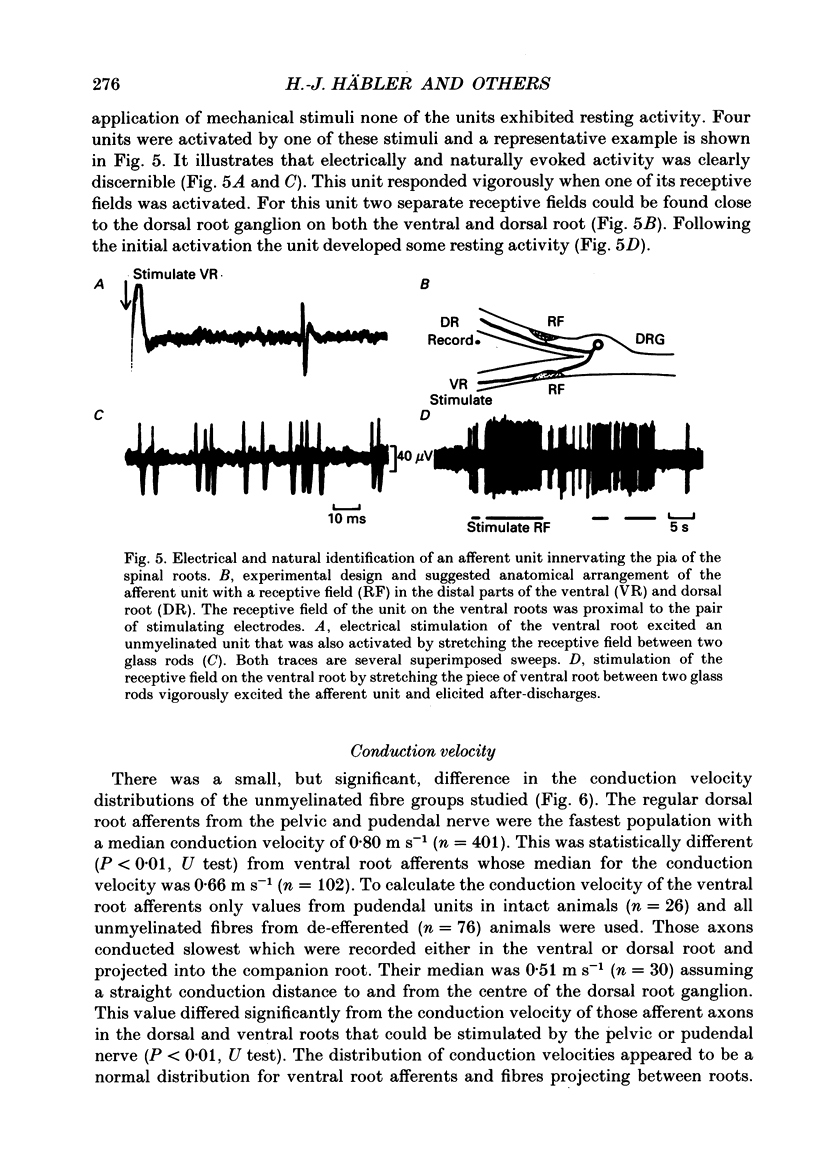
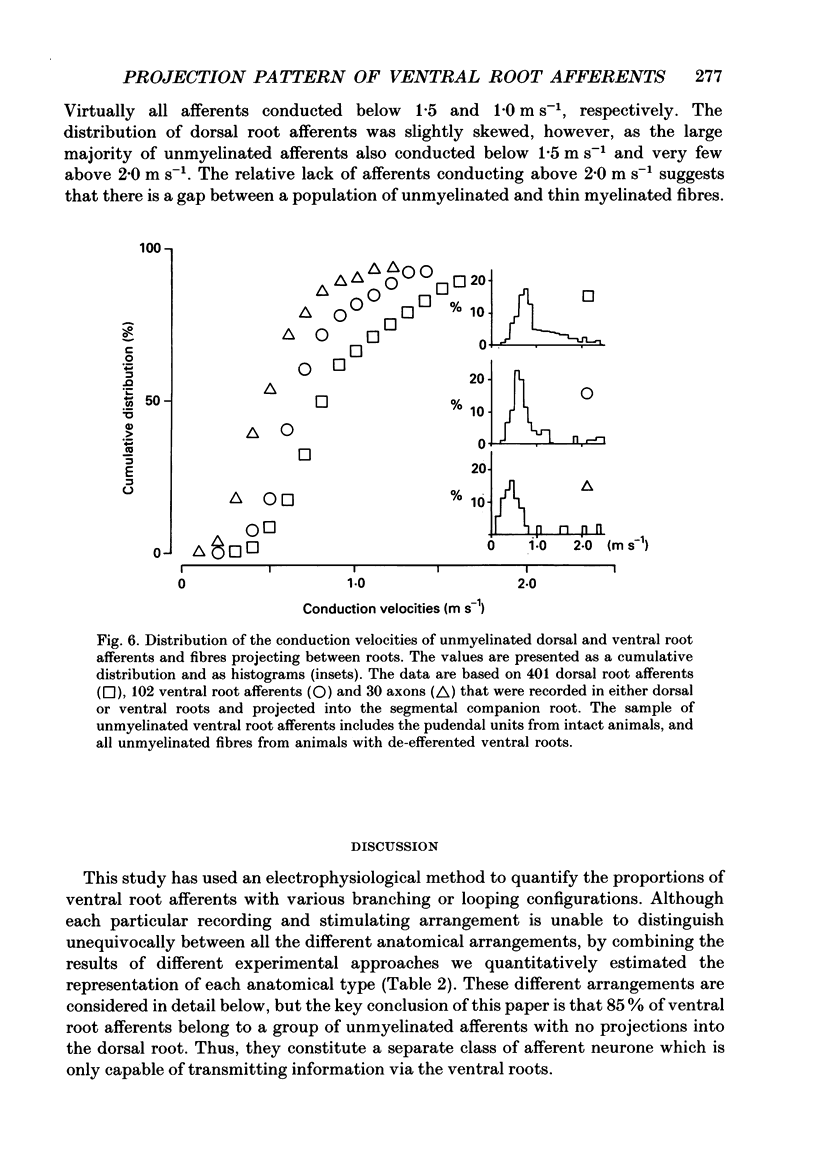
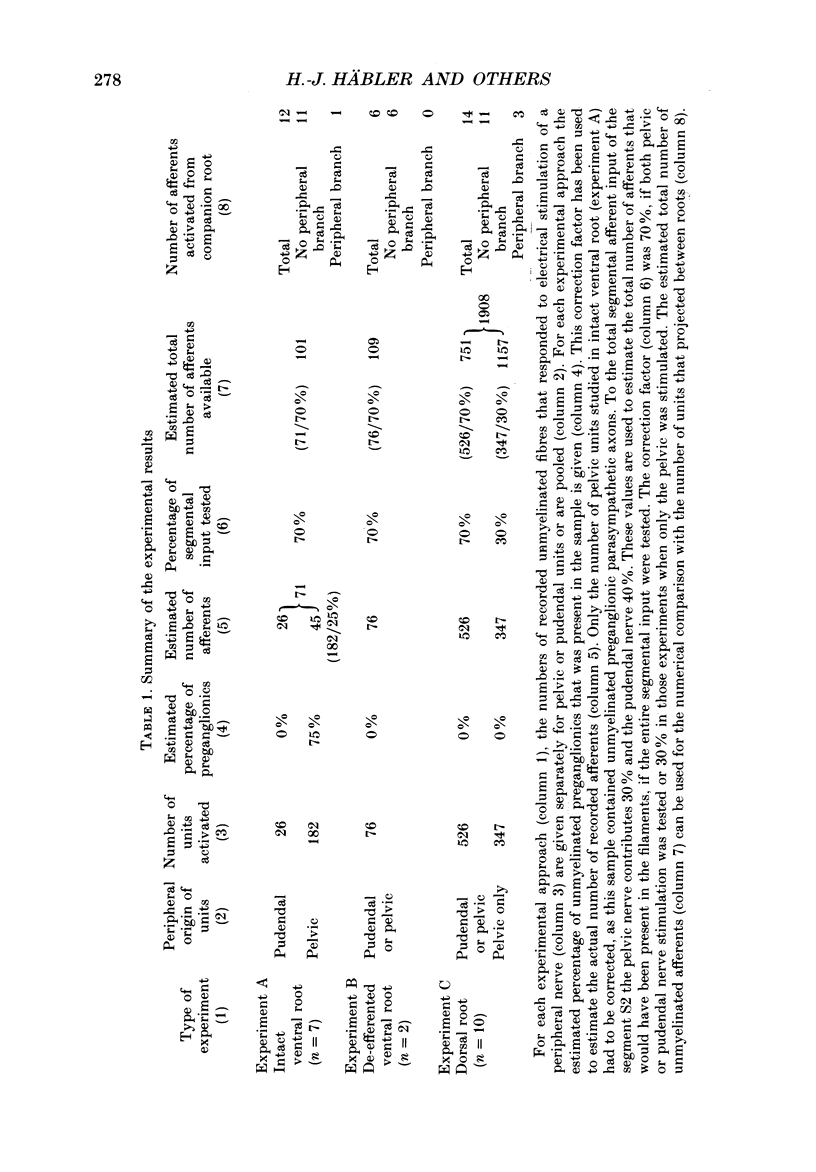









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum M. L., Clifton G. L., Coggeshall R. E., Coulter J. D., Vance W. H., Willis W. D. Unmyelinated fibres in the sacral 3 and caudal 1 ventral roots of the cat. J Physiol. 1976 Apr;256(3):557–572. doi: 10.1113/jphysiol.1976.sp011339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azerad J., Hunt C. C., Laporte Y., Pollin B., Thiesson D. Afferent fibres in cat ventral roots: electrophysiological and histological evidence. J Physiol. 1986 Oct;379:229–243. doi: 10.1113/jphysiol.1986.sp016250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie M. S., Bresnahan J. C., Mawe G. M., Finn S. Distribution and ultrastructure of ventral root afferents to lamina I of the cat sacral spinal cord. Neurosci Lett. 1987 Apr 23;76(1):1–6. doi: 10.1016/0304-3940(87)90182-0. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. IV. Unmyelinated fibres in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:61–72. [PubMed] [Google Scholar]
- Chung J. M., Kim J., Shin H. K. Blood pressure response evoked by ventral root afferent fibres in the cat. J Physiol. 1986 Jan;370:255–265. doi: 10.1113/jphysiol.1986.sp015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. M., Lee K. H., Kim J., Coggeshall R. E. Activation of dorsal horn cells by ventral root stimulation in the cat. J Neurophysiol. 1985 Aug;54(2):261–272. doi: 10.1152/jn.1985.54.2.261. [DOI] [PubMed] [Google Scholar]
- Chung K., Coggeshall R. E. The ratio of dorsal root ganglion cells to dorsal root axons in sacral segments of the cat. J Comp Neurol. 1984 May 1;225(1):24–30. doi: 10.1002/cne.902250104. [DOI] [PubMed] [Google Scholar]
- Chung K., Kang H. S. Dorsal root ganglion neurons with central processes in both dorsal and ventral roots in rats. Neurosci Lett. 1987 Sep 23;80(2):202–206. doi: 10.1016/0304-3940(87)90654-9. [DOI] [PubMed] [Google Scholar]
- Clifton G. L., Coggeshall R. E., Vance W. H., Willis W. D. Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol. 1976 Apr;256(3):573–600. doi: 10.1113/jphysiol.1976.sp011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E., Coulter J. D., Willis W. D., Jr Unmyelinated axons in the ventral roots of the cat lumbosacral enlargement. J Comp Neurol. 1974 Jan 1;153(1):39–58. doi: 10.1002/cne.901530105. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E. Law of separation of function of the spinal roots. Physiol Rev. 1980 Jul;60(3):716–755. doi: 10.1152/physrev.1980.60.3.716. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Risling M., Cuello C. Immunohistochemical localization of substance P in the lumbosacral spinal pia mater and ventral roots of the cat. Brain Res. 1982 Aug 19;246(1):168–171. doi: 10.1016/0006-8993(82)90159-7. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hendry I. A., Morton C. R., Hutchison W. D., Zhao Z. Q. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988 Jun 7;451(1-2):261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadić R., Vergara J., Alvarez J. Microtubules and caliber of central and peripheral processes of sensory axons. J Comp Neurol. 1985 Jun 8;236(2):258–264. doi: 10.1002/cne.902360209. [DOI] [PubMed] [Google Scholar]
- Fang X. B. The population of the dorsal root ganglion cells which have central processes in ventral root and their immunoreactivity. Brain Res. 1987 Feb 3;402(2):393–398. doi: 10.1016/0006-8993(87)90053-9. [DOI] [PubMed] [Google Scholar]
- Floyd K., Koley J., Morrison J. F. Proceedings: Afferent discharges in the sacral ventral roots of cats. J Physiol. 1976 Jul;259(1):37P–38P. [PubMed] [Google Scholar]
- Gibson S. J., Polak J. M., Anand P., Blank M. A., Yiangou Y., Su H. C., Terenghi G., Katagiri T., Morrison J. F., Lumb B. M. A VIP/PHI-containing pathway links urinary bladder and sacral spinal cord. Peptides. 1986;7 (Suppl 1):205–219. doi: 10.1016/0196-9781(86)90188-9. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Dichotomizing unmyelinated afferents supplying pelvic viscera and perineum are rare in the sacral segments of the cat. Neurosci Lett. 1988 Nov 22;94(1-2):119–124. doi: 10.1016/0304-3940(88)90281-9. [DOI] [PubMed] [Google Scholar]
- Kawatani M., Erdman S. L., de Groat W. C. Vasoactive intestinal polypeptide and substance P in primary afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1985 Nov 15;241(3):327–347. doi: 10.1002/cne.902410307. [DOI] [PubMed] [Google Scholar]
- Kawatani M., Nagel J., de Groat W. C. Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1986 Jul 1;249(1):117–132. doi: 10.1002/cne.902490109. [DOI] [PubMed] [Google Scholar]
- Kim J., Shin H. K., Chung J. M. Many ventral root afferent fibers in the cat are third branches of dorsal root ganglion cells. Brain Res. 1987 Aug 11;417(2):304–314. doi: 10.1016/0006-8993(87)90455-0. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Chung K., Chung J. M., Coggeshall R. E. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986 Jan 15;243(3):335–346. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- Light A. R., Metz C. B. The morphology of the spinal cord efferent and afferent neurons contributing to the ventral roots of the cat. J Comp Neurol. 1978 Jun 1;179(3):501–515. doi: 10.1002/cne.901790304. [DOI] [PubMed] [Google Scholar]
- Maynard C. W., Leonard R. B., Coulter J. D., Coggeshall R. E. Central connections of ventral root afferents as demonstrated by the HRP method. J Comp Neurol. 1977 Apr 15;172(4):601–608. doi: 10.1002/cne.901720404. [DOI] [PubMed] [Google Scholar]
- Morgan C., Nadelhaft I., de Groat W. C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981 Sep 20;201(3):415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I., Degroat W. C., Morgan C. Location and morphology of parasympathetic preganglionic neurons in the sacral spinal cord of the cat revealed by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1980 Sep 1;193(1):265–281. doi: 10.1002/cne.901930118. [DOI] [PubMed] [Google Scholar]
- Risling M., Dalsgaard C. J., Cukierman A., Cuello A. C. Electron microscopic and immunohistochemical evidence that unmyelinated ventral root axons make u-turns or enter the spinal pia mater. J Comp Neurol. 1984 May 1;225(1):53–63. doi: 10.1002/cne.902250107. [DOI] [PubMed] [Google Scholar]
- Risling M., Dalsgaard C. J., Terenius L. Neuropeptide Y-like immunoreactivity in the lumbosacral pia mater in normal cats and after sciatic neuroma formation. Brain Res. 1985 Dec 9;358(1-2):372–375. doi: 10.1016/0006-8993(85)90987-4. [DOI] [PubMed] [Google Scholar]
- Risling M., Hildebrand C., Aldskogius H. Postnatal increase of unmyelinated axon profiles in the feline ventral root L7. J Comp Neurol. 1981 Sep 20;201(3):343–351. doi: 10.1002/cne.902010304. [DOI] [PubMed] [Google Scholar]
- Risling M., Hildebrand C. Occurrence of unmyelinated axon profiles at distal, middle and proximal levels in the ventral root L7 of cats and kittens. J Neurol Sci. 1982 Nov;56(2-3):219–231. doi: 10.1016/0022-510x(82)90144-7. [DOI] [PubMed] [Google Scholar]
- Stevens R. T., Hodge C. J., Jr, Apkarian A. V. Catecholamine varicosities in cat dorsal root ganglion and spinal ventral roots. Brain Res. 1983 Feb 14;261(1):151–154. doi: 10.1016/0006-8993(83)91295-7. [DOI] [PubMed] [Google Scholar]
- Vergara I., Oberpaur B., Alvarez J. Ventral root nonmedullated fibers: proportion, calibers, and microtubular content. J Comp Neurol. 1986 Jun 22;248(4):550–554. doi: 10.1002/cne.902480407. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Takahashi K., Satomi H., Ise H. Origins of primary afferent fibers in the spinal ventral roots in the cat as demonstrated by the horseradish peroxidase method. Brain Res. 1977 May 6;126(2):350–354. doi: 10.1016/0006-8993(77)90731-4. [DOI] [PubMed] [Google Scholar]


