Abstract
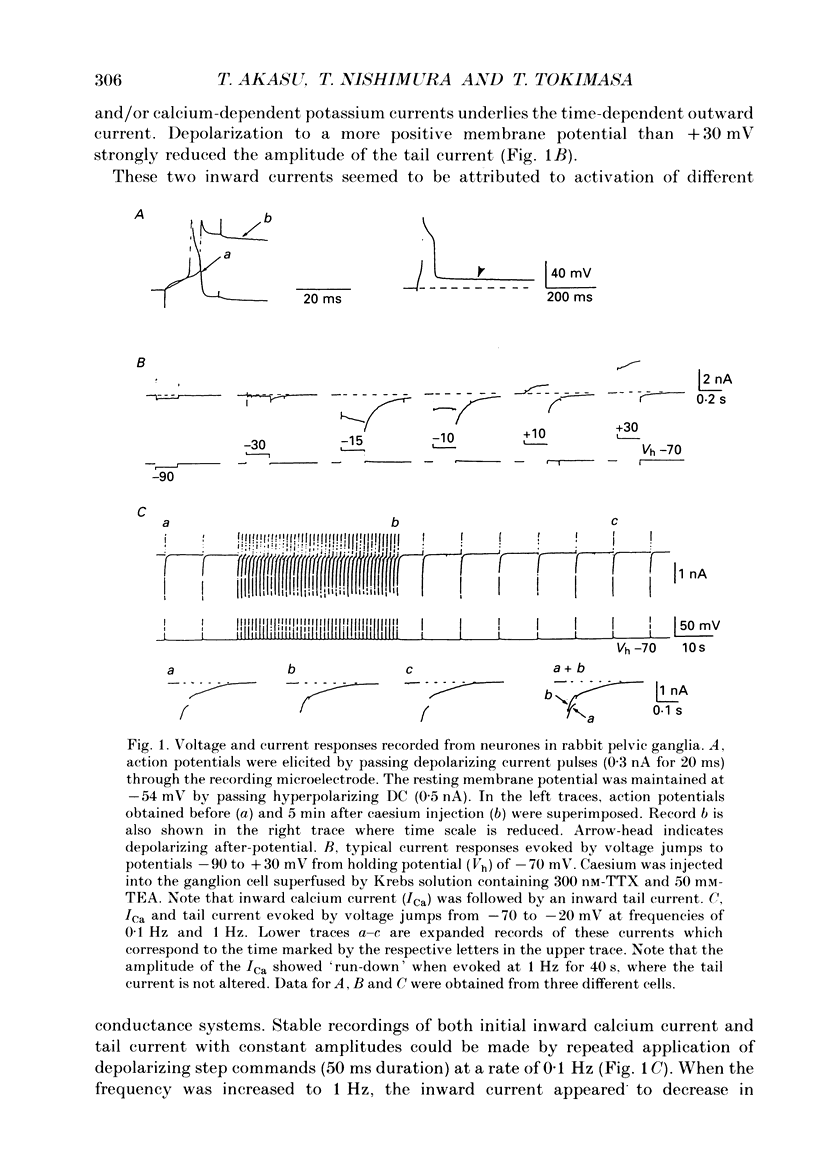
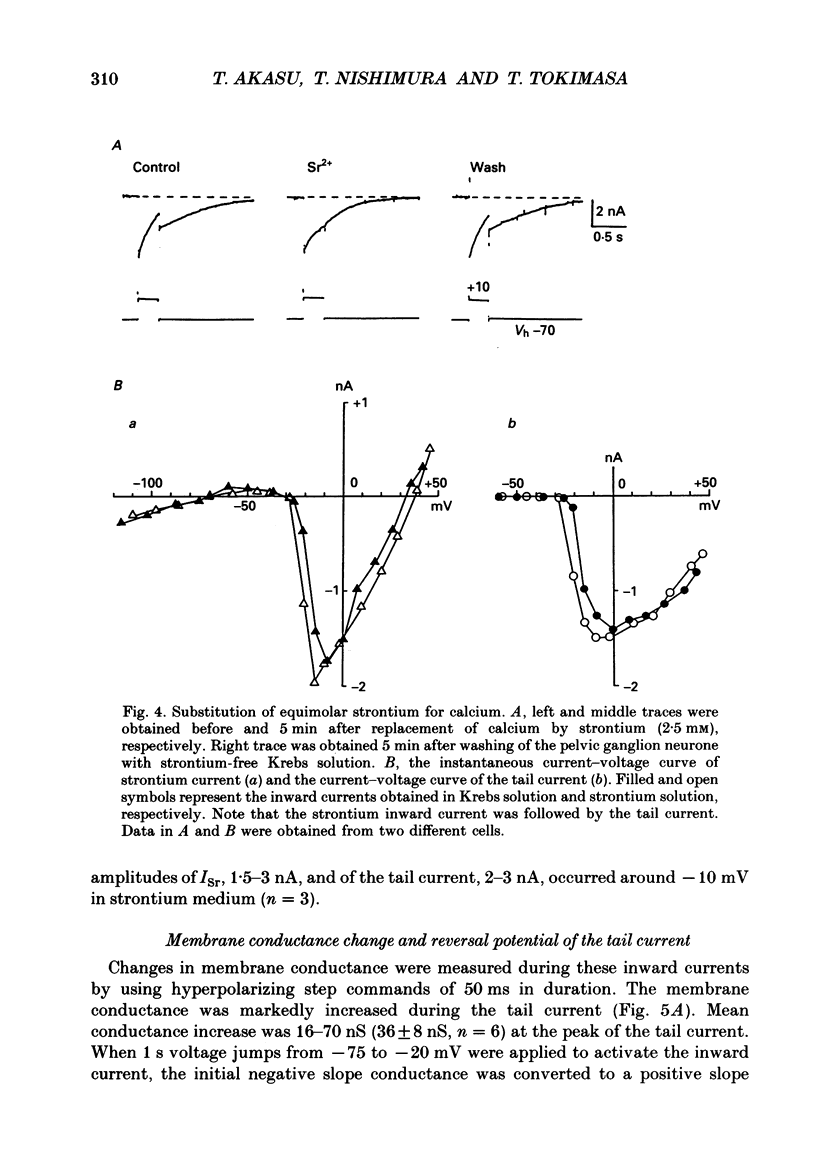
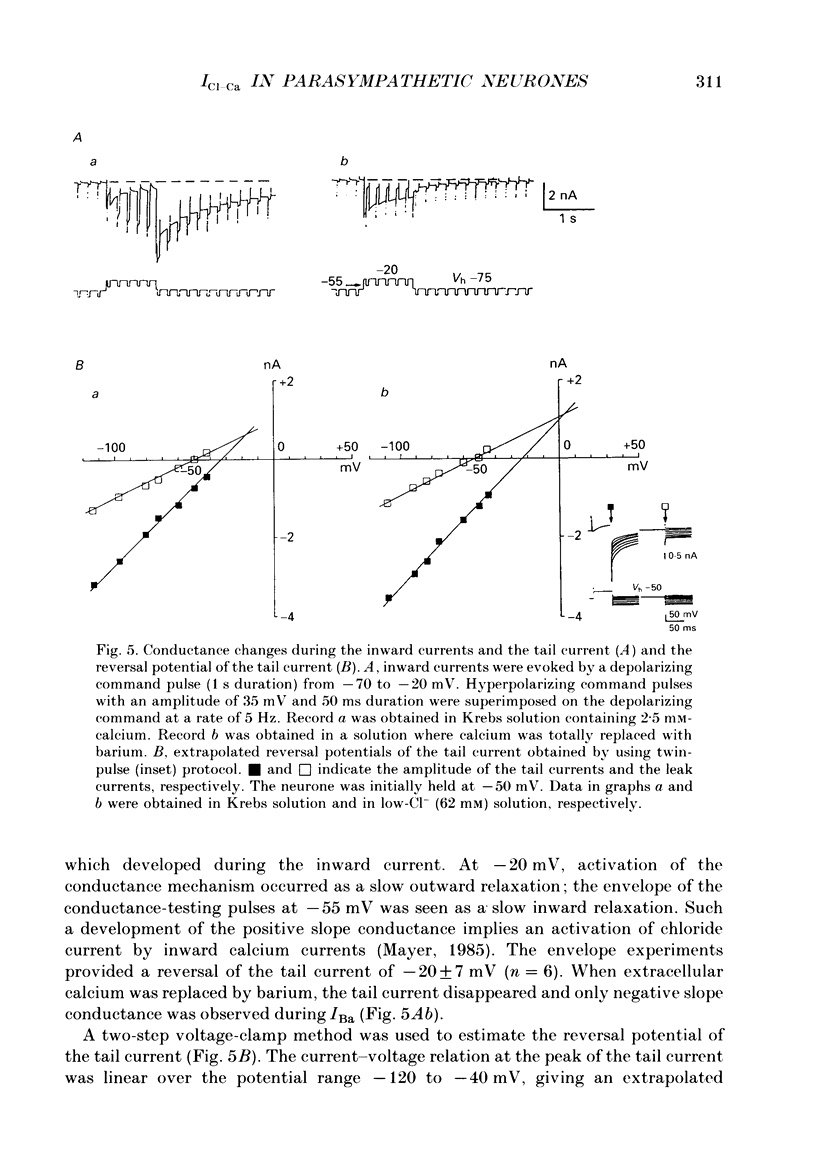
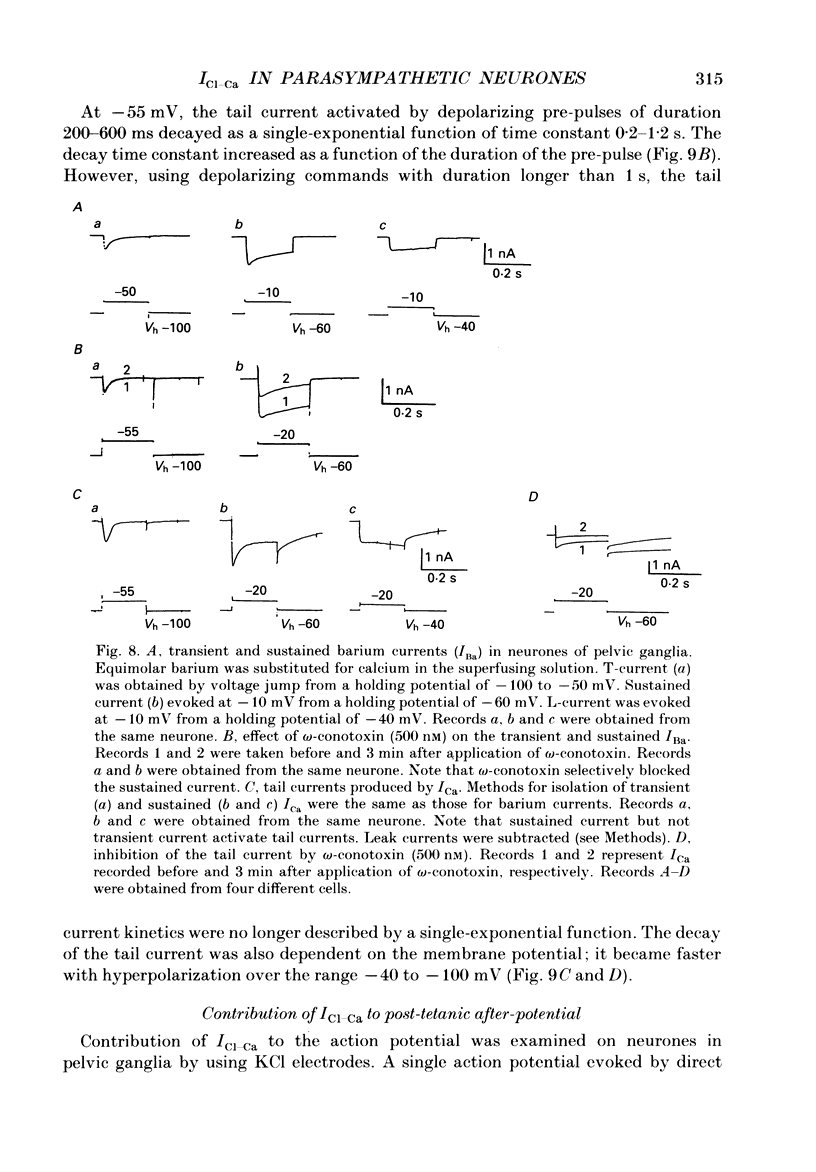
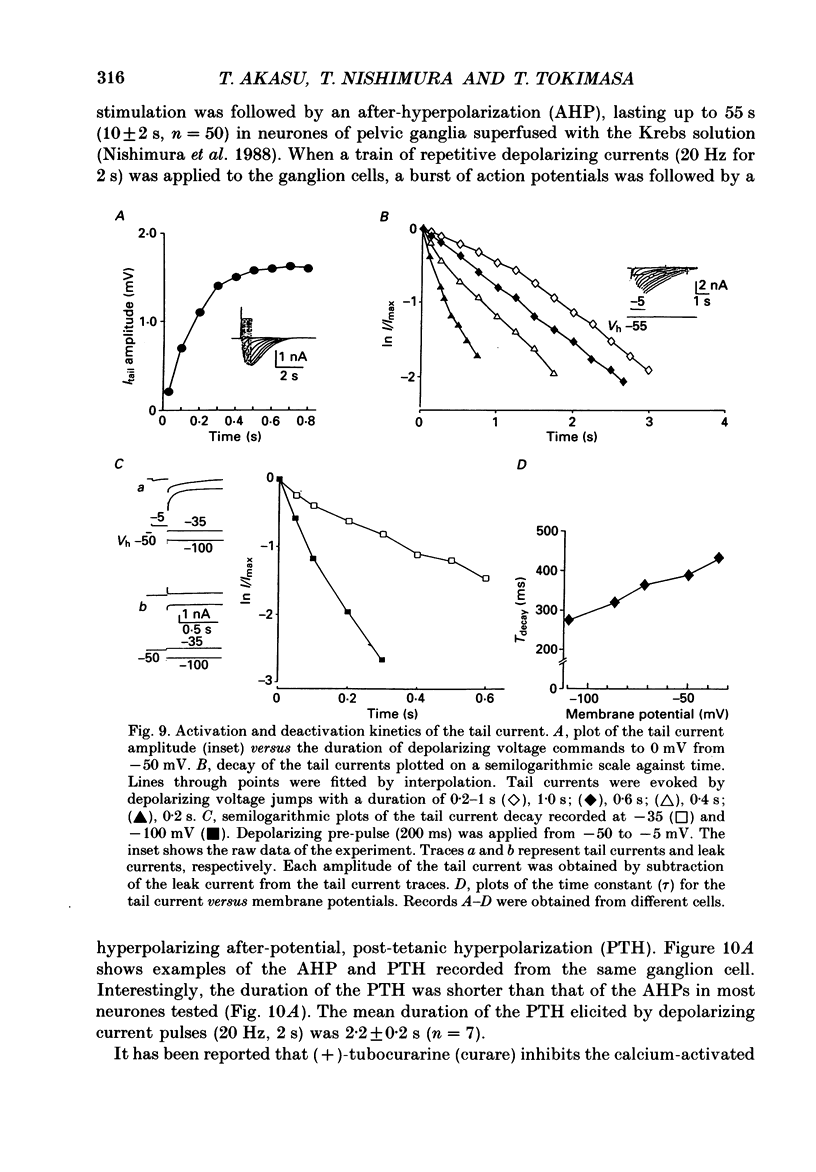
1. Voltage-clamp recordings were made from neurones in rabbit vesical pelvic ganglia by using single microelectrodes filled with 2 M-caesium chloride. Neurones were superfused with Krebs solution containing 300 nM-tetrodotoxin and 50 mM-tetraethylammonium. 2. Depolarizing voltage jumps activated inward currents followed by slowly decaying inward tail currents at -30 to +30 mV, which were accompanied by a large increase in membrane conductance. Both the inward current and tail current were blocked by cobalt (2 mM) or in a Krebs solution containing zero calcium and 12 mM-magnesium. 3. Substitution of barium for calcium enhanced the inward current, while it strongly reduced the tail current. Strontium substitution still exhibited both the inward current and the tail current. 4. Lowering external chloride activity increased the tail current amplitudes without affecting an initial calcium current. The reversal potentials of the tail current, measured using a twin-pulse protocol, were -18 +/- 5 mV (mean +/- S.E.M., n = 8) and +5 +/- 3 mV (n = 5) in Krebs solution and low-chloride (62 mM) solution, respectively, suggesting a calcium-dependent chloride current. 5. Stilbene derivatives, 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS, 0.01-1 mM) and 4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS, 0.01-1 mM), reversibly and concentration dependently depressed the tail current without affecting the calcium current. 6. Transient (T) and sustained (N and L) types of calcium current were likely to co-exist in neurones of the rabbit pelvic ganglia. Calcium-dependent chloride current was activated by N- and L-type calcium currents but not by T-type current. 7. Activation of the tail current at 0 to +20 mV was described by a single-exponential function. The tail current decayed exponentially at a holding membrane potential of -70 mV. Tail decay time constants were dependent on voltage and duration of the step command. 8. Substantial activation of the calcium-dependent chloride conductance could occur during a post-tetanic after-potential when pelvic ganglia neurones fired action potentials repetitively.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
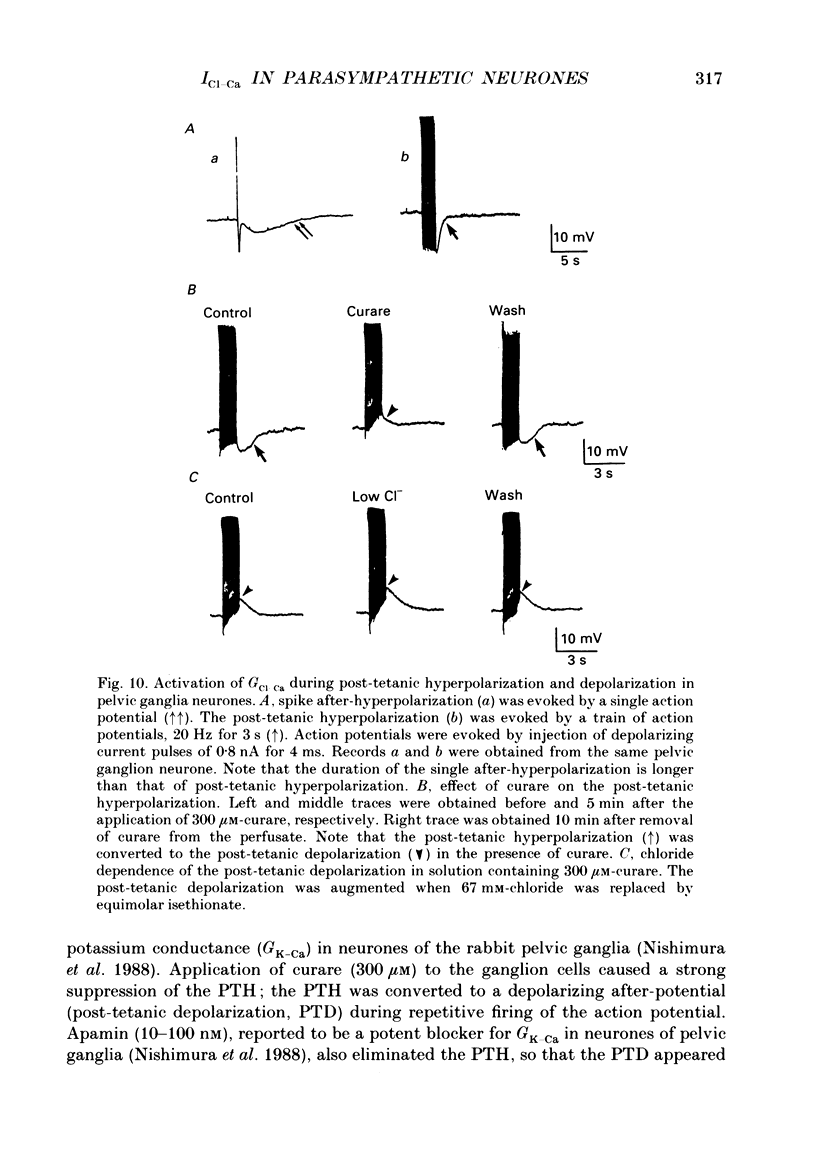
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Weight F. F. Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol. 1987 Dec;58(6):1431–1451. doi: 10.1152/jn.1987.58.6.1431. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Shuba YaM, Savchenko A. N. Three types of calcium channels in the membrane of mouse sensory neurons. Pflugers Arch. 1988 Jun;411(6):661–669. doi: 10.1007/BF00580863. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S. Calcium ions and the post-tetanic hyperpolarization of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1974 Oct;24(5):501–512. doi: 10.2170/jjphysiol.24.501. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Tokimasa T., Akasu T. Calcium-dependent potassium conductance in neurons of rabbit vesical pelvic ganglia. J Auton Nerv Syst. 1988 Sep;24(1-2):133–145. doi: 10.1016/0165-1838(88)90142-7. [DOI] [PubMed] [Google Scholar]
- North R. A. Electrophysiology of the enteric nervous system. Neuroscience. 1982 Feb;7(2):315–325. doi: 10.1016/0306-4522(82)90269-x. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Inoue K., Suzuki S., Barker J. L. A slow calcium-dependent chloride conductance in clonal anterior pituitary cells. J Neurophysiol. 1988 Jun;59(6):1854–1870. doi: 10.1152/jn.1988.59.6.1854. [DOI] [PubMed] [Google Scholar]
- Scott R. H., McGuirk S. M., Dolphin A. C. Modulation of divalent cation-activated chloride ion currents. Br J Pharmacol. 1988 Jul;94(3):653–662. doi: 10.1111/j.1476-5381.1988.tb11572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Nishimura T., Akasu T. Calcium-activated chloride conductance in parasympathetic neurons of the rabbit urinary bladder. J Auton Nerv Syst. 1988 Sep;24(1-2):123–131. doi: 10.1016/0165-1838(88)90141-5. [DOI] [PubMed] [Google Scholar]


