Abstract
Engagement of the T-cell receptor (TCR) triggers a series of signaling events that lead to the activation of T cells. HIP-55 (SH3P7 or mAbp1), an actin-binding adaptor protein, interacts with and is tyrosine phosphorylated by ZAP-70, which is a crucial proximal protein tyrosine kinase for TCR signaling. HIP-55 is important for JNK and HPK1 activation induced by TCR signaling. In this study, we report the generation and characterization of HIP-55 knockout mice. We found that HIP-55 knockout mice were viable and fertile but showed decreased body weight and increased occurrence of death within the first 4 weeks after birth. The lymphoid organs in HIP-55 knockout mice showed cellularity and T-cell development comparable to that of the wild-type mice. HIP-55 knockout T cells displayed defective T-cell proliferation, decreased cytokine production, and decreased up-regulation of the activation markers induced by TCR stimulation. TCR internalization was slightly increased in HIP-55 knockout T cells. These phenotypes were accompanied by reduced immune responses, including antigen-specific antibody production and T-cell proliferation in HIP-55 knockout mice. The TCR-induced signaling events, including LAT/phospholipase Cγ1 phosphorylation and HPK1/JNK activation, were partially defective in HIP-55 knockout T cells. These results demonstrate the importance of HIP-55 as an adaptor protein in the TCR signaling and immune system.
The activation of T lymphocytes plays a central role in the regulation of immune responses. An optimal T-cell response requires signals delivered from the T-cell receptor (TCR) and coreceptors. Engagement of the TCR triggers the activation of Src family protein tyrosine kinases Lck and Fyn, which subsequently phosphorylate the immunoreceptor tyrosine-based activation motifs in the CD3ζ tail. The phosphorylated tyrosine-based activation motifs recruit ZAP-70 through the Src homology 2 (SH2) domain of ZAP-70. The recruitment of ZAP-70 to the TCR complex leads to the activation of ZAP-70 by Lck. Upon activation, ZAP-70 plays a major role in propagating the TCR signals to downstream molecules, including LAT, phospholipase Cγ1 (PLCγ1), and SLP-76. LAT is localized in specific plasma membrane compartments known as glycolipid-enriched microdomains and serves as a docking protein for other signaling molecules, including SLP-76 and Grb2. The LAT-associated adaptors then recruit various signaling molecules to the glycolipid-enriched microdomains and promote multiple downstream signaling events, such as mitogen-activated protein kinase activation, the release of intracellular Ca2+, and increased interleukin-2 production (1, 2, 16, 19, 21, 28).
HIP-55 (hematopoietic progenitor kinase 1 [HPK1]-interacting protein of 55 kDa; also called SH3P7 and mAbp1) is a novel SH3 domain-containing protein (11). HIP-55 contains an actin-binding domain at its N terminus and an SH3 domain at its C terminus (11), and it binds to filamentous actin and colocalizes with actin filaments (17, 20). HIP-55 also interacts with dynamin and Cdc42, which is important for receptor-mediated endocytosis and Golgi transport (13, 18, 27). HIP-55 is cleaved during apoptosis (6). HIP-55 interacts with HPK1 (11), a serine/threonine protein kinase involved in TCR signaling (15, 23, 24). Upon TCR stimulation, HIP-55 translocates to glycolipid-enriched microdomains (14, 22) and binds to phosphorylated ZAP-70 (14). In HIP-55-knockdown Jurkat T cells established by RNA interference, the signaling events induced by TCR stimulation, such as HPK1 and JNK activation, are defective (14), suggesting that HIP-55 may be an important regulator in TCR signaling.
To study the in vivo functions of HIP-55 in TCR signaling and the immune system, we generated and characterized HIP-55 knockout mice. We found that HIP-55 knockout mice showed decreased body weight and higher occurrence of death within the first 4 weeks after birth. The cellularity of lymphoid organs and the development of T cells in HIP-55 knockout mice were normal. However, HIP-55 knockout T cells were less responsive to TCR stimulation, as manifested here by reduced T-cell proliferation, lower cytokine production, and decreased up-regulation of activation markers. Additionally, antigen-specific immune responses were also reduced in HIP-55 knockout mice. The signaling events including LAT/PLCγ1 phosphorylation and HPK1/JNK activation induced by TCR stimulation were partially decreased in HIP-55 knockout T cells, which may be responsible for the defects we observed in the immune system. These findings provide evidence that HIP-55 is an important adaptor protein that regulates T-cell activation and immune responses.
MATERIALS AND METHODS
Generation of HIP-55 knockout mice.
A mouse HIP-55 knockout embryonic stem cell clone (BayGenomics, Davis, CA) was injected into C57BL/6 blastocysts to generate chimeric mice. The heterozygous HIP-55 progeny from the crossing of the chimeras with C57BL/6 mice were used to generate HIP-55 knockout mice. The genotype was determined by Southern blotting and PCR. Mice used in this study were 6 to 10 weeks old with a mixed C57BL/6 × 129/Ola genetic background. Mice were backcrossed to C57BL/6 for eight generations during the time of analysis. The data presented here reflect experiments performed on sex-matched littermates. Animals were housed under specific-pathogen-free conditions under institutional guidelines, and all mouse protocols were approved by Baylor College of Medicine.
Flow cytometry and purification of T cells.
Thymocytes, splenocytes, and lymph node cells were dissected and crushed in phosphate-buffered saline containing 3% fetal bovine serum. Red blood cells were depleted from samples. Debris was removed by straining. Cells were stained with different fluorescently labeled antibodies as indicated and analyzed on a FACSCaliber (Becton Dickinson). To obtain T cells (>90% pure), red blood cell-depleted splenocytes were isolated and negatively selected using a cocktail of anti-B220, anti-Gr-1, anti-CD11b, and anti-Ter119 antibodies (Pharmingen) on an AutoMACs (Miltenyi Biotech).
T-cell proliferation and stimulation assays.
Splenocytes or lymph node cells were plated at a density of 2 × 105 cells per well in flat-bottomed 96-well plates. Cells were unstimulated or stimulated with anti-CD3 (145-2C11, Pharmingen) at different concentrations as indicated or with 20 ng/ml phorbol myristate acetate and 0.1 μM ionomycin. Cells were cultured for 48 h and labeled with 1 μCi/well [3H]thymidine for 16 h. Thymidine incorporation was then measured with a scintillation counter. Cytokine production was measured using the kit from BD Biosciences. For T-cell stimulation, lymph node cells or purified T cells were incubated for 15 min at 4°C with 10 μg/ml biotin-conjugated anti-CD3 (500A2, Pharmingen), incubated for 10 min at 4°C with 10 μg/ml streptavidin (Sigma), and placed in a 37°C water bath for the indicated times. Jurkat T-cell stimulation using an anti-CD3 antibody (OKT3) was performed as previously described (14).
Immunoprecipitation and Western blotting.
Cells were lysed in ice-cold 1% Triton X-100 lysis buffer (150 mM NaCl, 20 mM HEPES [pH 7.4], 2 mM EGTA, 50 mM glycerophosphate, 10% glycerol, 1% Triton X-100, 0.5% Nonidet P-40) freshly supplemented with 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 3 μg/ml aprotinin. The cell lysate was incubated with the indicated antibodies for 2 h or overnight and protein G-Sepharose beads for 1 h at 4°C. The immunocomplexes were washed four times with lysis buffer and then subjected to Western blot analysis. For Western blotting, immunocomplexes or cellular proteins (50 to 100 μg) were resolved by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and the gels were transferred to polyvinylidene difluoride membranes at 4°C. Western blotting was performed as previously described using the indicated antibodies (14).
Immunocomplex kinase assays.
JNK and HPK1 immunocomplex kinase assays were performed as previously described (7, 8). Glutathione S-transferase (GST)-c-Jun and myelin basic protein were used as the substrate for the JNK and HPK1 kinase assays, respectively.
Animal immunization.
For in vivo T-cell activation, mice were immunized subcutaneously at the base of the tail with 4-hydroxy-3-nitrophenylacetyl (NP) coupled to chicken γ globulin (NP-CGG) (5 μg/g mouse weight) emulsified in complete Freund's adjuvant (Sigma). Cells were prepared from the enlarged draining lymph nodes 7 days later and restimulated with different doses of NP-CGG. Cells were cultured for 64 h and then labeled with 1 μCi/well [3H]thymidine for 16 h. Thymidine incorporation was then measured with a scintillation counter.
For T-cell-mediated humoral immune responses, mice were immunized by intraperitoneal injection of alum-precipitated NP-CGG in phosphate-buffered saline (5 μg/g mouse weight). Thirty-one days after the first immunization, mice were reinjected with NP-CGG in phosphate-buffered saline (2.5 μg/g mouse weight). Serum was collected at indicated times (days 7, 14, 21, and 30 after the first immunization and day 5 after the second immunization). The titers of anti-NP-specific immunoglobulin G1 (IgG1) and IgM in the serum were measured by enzyme-linked immunosorbent assay (ELISA) using NP-bovine serum albumin as the coating antigen, followed by incubation with anti-IgG1 and anti-IgM goat anti-mouse antibodies.
Generation of HIP-55 shRNA-knockdown Jurkat T cells.
To generate HIP-55 small-hairpin RNA (shRNA), a 19-nucleotide sequence (GGTGCTGGCTCTGAGCACA) selected from human HIP-55 cDNA (1060 to 1078) was annealed and ligated into pSUPER vector (14). HIP-55 shRNA was stably transfected into Jurkat T cells, and HIP-55 shRNA-knockdown Jurkat T cells were generated as described (14).
TCR internalization.
For CD3ɛ internalization, splenic T cells were mixed with biotin-anti-CD3ɛ (10 μg/ml) on ice for 30 min, and then the cells were either stopped with 0.1% NaN3 or incubated in 37°C for 2 h. Then the internalization was stopped with 0.1% NaN3 and the cells were stained with Tricolor-streptavidin and anti-CD4-fluorescein isothiocyanate. For TCRβ internalization, splenic T cells were treated with prebound anti-CD3ɛ (10 μg/ml) for 0, 2, and 4 h, then the internalization was stopped with 0.1% NaN3 and the cells were stained with anti-CD4-fluorescein isothiocyanate and anti-TCRβ-phycoerythrin.
Antibodies.
The anti-HIP-55 (N terminus, Ab571) and anti-JNK antibodies were previously described (14). The anti-HIP-55 (C terminus, amino acids 406 to 436) was a generous gift from M. Stamnes (University of Iowa, Iowa City, IA). The anti-HPK1 (Ab870) antibody was generated against a peptide corresponding to mouse HPK1 amino acids 473 to 490. The anti-CD4-fluorescein isothiocyanate, anti-CD8-phycoerythrin, anti-CD3-fluorescein isothiocyanate, anti-B220-phycoerythrin, anti-TCR-β, anti-CD3ɛ-biotin, anti-CD44-phycoerythrin, anti-CD25-biotin, and anti-CD69-phycoerythrin were purchased from BD Biosciences (San Diego, CA). The anti-phospho-p38 antibody was from Cell Signaling Technology (Beverly, MA). The anti-PLCγ1 (1249) and anti-p38 antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-LAT antibody and antiphosphotyrosine monoclonal antibody (4G10) were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-IgG1 and anti-IgM goat anti-mouse antibodies were from Southernbiotech (Birmingham, AL).
RESULTS
Generation of HIP-55 knockout mice.
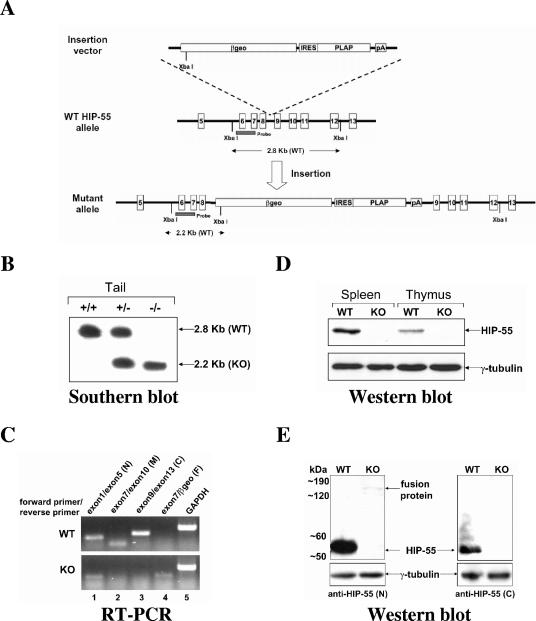
To study the functions of HIP-55 in TCR signaling, we generated HIP-55 knockout mice using a gene-trapping strategy. An insertion vector containing β-geo (a fusion between the β-galactosidase and neomycin phosphotransferase genes), human placental alkaline phosphatase, and an internal ribosome entry site was inserted in the intron between exons 8 and 9 of the targeted Hip-55 allele (Fig. 1A). After the insertion, a fusion transcript was generated through splicing of the region from exons 1 to 8 of the Hip-55 gene to β-geo (Fig. 1A). The functional domains of HIP-55, including consensus tyrosine phosphorylation sites Tyr-334/344 and the SH3 domain (amino acids 374 to 431) were absent in the expressed fusion protein. Therefore, we predicted that the expressed product would not be functional. Successful targeting was confirmed by Southern blotting analysis of DNA from targeted embryonic stem cells and from the progeny of “heterozygous-by-heterozygous” mating (Fig. 1B).
FIG. 1.
Generation of HIP-55 knockout mice. (A) Structures of the insertion vector, wild-type (WT) allele and mutant allele. The XbaI sites, probe region, and sizes of the bands expected in Southern blot analysis are indicated. βgeo, a fusion between the β-galactosidase and neomycin phosphotransferase genes; PLAP, human placental alkaline phosphatase; IRES, internal ribosome entry site. (B) Southern blot analysis of XbaI-digested genomic DNA from wild-type (WT), heterozygous, or homozygous mice using the probe indicated in A. The 2.8-kb and 2.2-kb bands represent the wild-type and mutant alleles, respectively. (C) Reverse transcription-PCR analysis of splenic cells from wild-type (WT) and HIP-55 knockout (KO) mice using primers as indicated (N, N terminus; M, middle part; C, C terminus; F, fusion part). (D) Western blot analysis of splenic and thymic cell lysates from wild-type (WT) and HIP-55 knockout (KO) mice using anti-HIP-55 (N-terminal) and anti-γ-tubulin antibodies. (E) Western blot analysis of splenic cell lysates from wild-type (WT) and HIP-55 knockout (KO) mice using anti-HIP-55 (N-terminal and C-terminal) and anti-γ-tubulin antibodies.
Reverse transcription-PCR analysis using primers to the N terminus, middle part, and C terminus of HIP-55 demonstrated that there were no mRNA products for the middle part (Fig. 1C, lane 2) and C terminus (Fig. 1C, lane 3) in HIP-55 knockout mice. Though detectable, the level of the N-terminal mRNA product in knockout mice was much lower than that of the wild type (Fig. 1C, lane 1). Western blotting analysis using an antibody recognizing the N-terminal peptide sequence amino acids 172 to 187 of HIP-55 confirmed the complete loss of native HIP-55 expression in the thymus and spleen of the HIP-55 knockout mice (Fig. 1D).
A fusion protein of truncated HIP-55 and β-geo was barely detectable in the splenic cell lysate from HIP-55 knockout mice by Western blotting analysis, and the protein expression level was extremely low and less than 0.5% of the native HIP-55 expression (Fig. 1E). This fusion protein was not detectable using an anti-C-terminal HIP-55 antibody (Fig. 1E), suggesting that the fusion protein contains only the N-terminal HIP-55. As shown by our RNA and protein analyses, the gene trapping strategy disrupted the normal transcription and translation of native HIP-55 and caused a phenotypically null allele and HIP-55 deficiency in mice.
HIP-55 knockout mice are viable and fertile but show lower body weight and high occurrence of death.
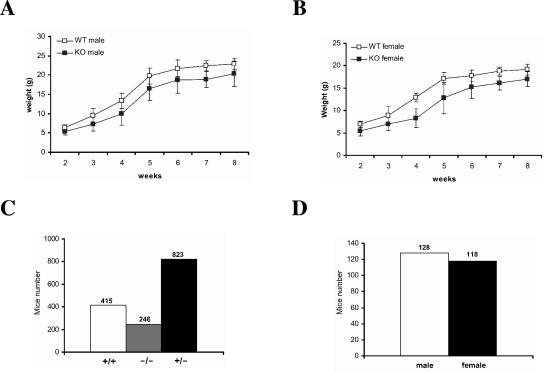
The HIP-55 knockout mice were viable and fertile. However, both male and female HIP-55 knockout mice showed lower body weight compared to the wild-type littermates (Fig. 2A and B). Moreover, the HIP-55 knockout mice showed some unhealthy symptoms, including slightly trembling hind limbs and partial tail paralysis. The offspring produced from breeding among HIP-55 heterozygous mice did not show Mendelian ratios. Of 1,484 viable offspring, 415 (28.0%) were wild type, 823 (55.5%) were heterozygous, and only 246 (16.6%) were HIP-55 homozygous knockout mice (Fig. 2C). Among the viable HIP-55 knockout mice, the numbers of male and female mice were comparable (Fig. 2D). Our data indicate that HIP-55 knockout mice have decreased body weight (Fig. 2A and B), which may cause the high occurrence of death.
FIG. 2.
Body weight and high occurrence of death in HIP-55 knockout mice. (A and B) The body weights of male (A) and female (B) wild-type (WT) and HIP-55 knockout (KO) mice were measured at the indicated time points. Each group represents 10 to 15 mice. (C) The numbers of wild-type, knockout, and heterozygous offspring from heterozygous-by-heterozygous mating were counted. (D) The numbers of male and female HIP-55 knockout offspring from heterozygous-by-heterozygous mating were counted.
Development of lymphoid organs is not affected by HIP-55 deficiency.
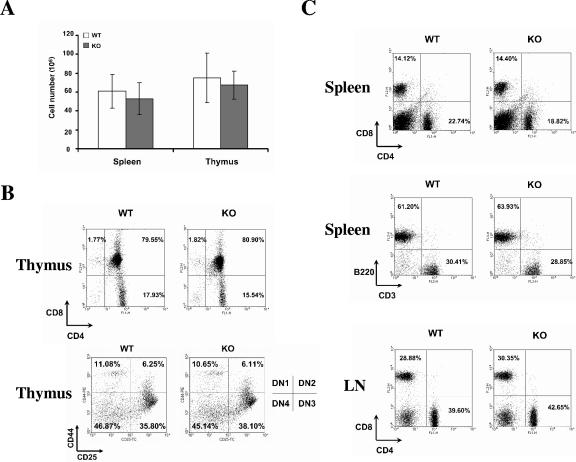
Although HIP-55 knockout mice showed a decrease in body weight, initial analysis of the HIP-55 knockout mice failed to identify significant alterations in the cellularity and development of lymphoid organs. There was a slight decrease of the cell numbers in the spleens and thymi of HIP-55 knockout mice compared to wild-type littermates (Fig. 3A). Both strains showed comparable ratios of thymic CD4+ CD8+, CD4+ CD8−, and CD4− CD8+ cells (Fig. 3B). Within the CD4 and CD8 double-negative population, the thymocytes can be further divided into four developmental stages based on CD25 and CD44 expressions; no obvious defect in the double-negative thymocyte development was observed in HIP-55 knockout mice (Fig. 3B). In the spleens and lymph nodes, there were similar proportions of CD4+ and CD8+ cells between wild-type and HIP-55 knockout mice (Fig. 3C). No obvious perturbation of the T- and B-cell ratio appeared in the spleens of HIP-55 knockout mice as measured by CD3/B220 staining (Fig. 3C). The data indicate that HIP-55 is not essential for the normal development of lymphoid cells.
FIG. 3.
Lymphoid organ development. (A) Total cellularity of spleen and thymus in wild-type (WT) and HIP-55 knockout (KO) mice. Data are representative of 15 6-week-old mice for each group. (B and C) Flow cytometric analysis of cells from the thymus (B) and spleen and lymph node (C). The antibodies used for staining and percentages of representative lymphoid populations are indicated. Data are representative of three experiments.
HIP-55 mediates TCR-induced T-cell proliferation, cytokine production, and activation marker up-regulation.
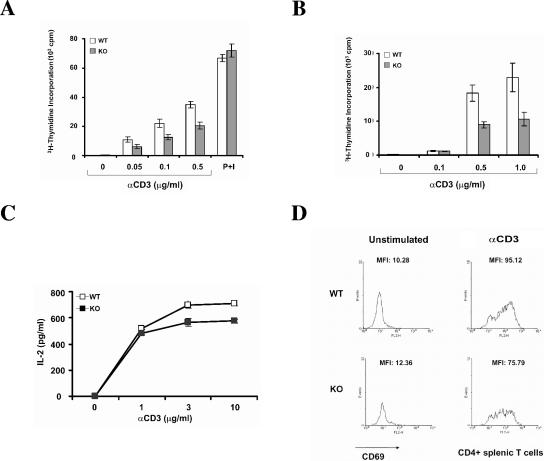
To assess the properties of lymphocytes lacking HIP-55, we examined the effect of HIP-55 deficiency on T-cell proliferation. Measured by [3H]thymidine incorporation, HIP-55 knockout splenic and lymph node T cells were markedly defective in their ability to proliferate in response to anti-CD3-mediated TCR signaling compared to the wild-type counterparts (Fig. 4A and B). Their proliferative response to a combination of phorbol 12-myristate 13-acetate and ionomycin was similar to that of wild-type T cells (Fig. 4A), which indicates that the defect in proliferation is upstream of protein kinase C and Ca2+ influx in the TCR signaling cascade and the general signaling remains intact. When we examined the interleukin-2 production of T cells after anti-CD3 stimulation, we found that HIP-55 knockout T cells showed lower cytokine production compared to wild-type T cells (Fig. 4C).
FIG. 4.
Defective TCR-induced proliferation, cytokine production, and up-regulation of activation marker in HIP-55 knockout T cells. (A and B) Splenic (A) and lymph node (B) T cells from wild-type (WT) and HIP-55 knockout (KO) mice were left unstimulated or stimulated with different concentrations of anti-CD3 antibody or phorbol myristate acetate (P; 20 ng/ml) and ionomycin (I; 0.1 μM) for 48 h, and then pulsed with [3H]thymidine for the last 16 h. Each assay was performed in triplicate wells, and data are representative of three experiments. (C) Purified splenic T cells from wild-type and HIP-55 knockout mice were left unstimulated or stimulated with different concentrations of prebound anti-CD3 antibody. The supernatant was collected after 64 h and the interleukin-2 was measured using ELISA. Data are representative of three independent experiments. The error bars are standard deviations from replicates of the representative experiment. The difference is statistically significant (P < 0.01). (D) Wild-type and HIP-55 knockout splenic T cells were stimulated with 1 μg/ml anti-CD3 antibody and cultured for 24 h. The cells were stained with anti-CD4-fluorescein isothiocyanate and anti-CD69-phycoerythrin and analyzed by FACSCaliber. Data are representative of the CD4+ population.
After TCR ligation, T cells undergo a series of signaling events, including cytokine secretion and up-regulation of cell surface activation markers. CD69 is a known activation marker that appears after TCR engagement and that indicates the activation status of T cells (5, 9). To further analyze the effect of HIP-55 deficiency on T-cell function, splenic T cells were stimulated with anti-CD3 for 24 h and stained with anti-CD69 to study the up-regulation of this T-cell activation marker. We found that the stimulated HIP-55 knockout T-cells expressed lower amounts of CD69 (Fig. 4D) on the cell surface compared to wild-type T cells, as indicated by the mean fluorescence intensity of stimulated CD4+ T cells. Together these results demonstrate that HIP-55 positively regulates T-cell proliferation, cytokine production, and activation marker up-regulation induced by TCR stimulation.
TCR internalization in HIP-55 knockout T cells.
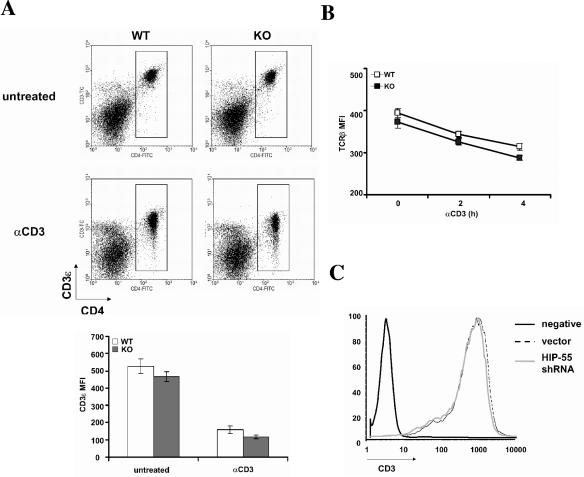
There is evidence that HIP-55 is involved in endocytosis (27). Using mainly an overexpression system, HIP-55 was shown to promote the down-regulation of TCR expression in Jurkat T cells (22). When we examined TCR internalization in HIP-55 knockout T cells, we observed only a slight decrease of TCRβ and CD3ɛ before and after TCR stimulation compared to wild-type cells (Fig. 5 A and B). Consistently, we also observed a minor decrease of CD3ɛ in the HIP-55 shRNA-transfected Jurkat T cells (Fig. 5C). The data indicate that HIP-55 is not essential for TCR internalization.
FIG. 5.
TCR internalization in HIP-55 knockout T cells. (A) Splenocytes from wild-type (WT) and HIP-55 knockout (KO) mice were unstimulated or stimulated with biotin-anti-CD3ɛ (10 μg/ml) for 2 h at 37°C and then stained with fluorescein isothiocyanate-labeled anti-CD4 antibody and Tricolor-labeled streptavidin. The mean fluorescence intensity in the lower panel represents the intensity of CD3ɛ in the CD4+ population. Data are derived from three mice in each group. (B) Splenocytes from wild-type (WT) and HIP-55 knockout (KO) mice were unstimulated or stimulated with prebound anti-CD3ɛ (10 μg/ml) for 2 or 4 h at 37°C and then stained with fluorescein isothiocyanate-labeled anti-CD4 and phycoerythrin-labeled anti-TCRβ antibodies. The mean fluorescence intensity represents the intensity of TCRβ in the CD4+ population. Data are derived from three mice in each group. (C) HIP-55 shRNA and vector-transfected Jurkat T cells were stained with an anti-CD3ɛ antibody. Negative stands for no staining (negative control).
Reduced immune responses in HIP-55 knockout mice.
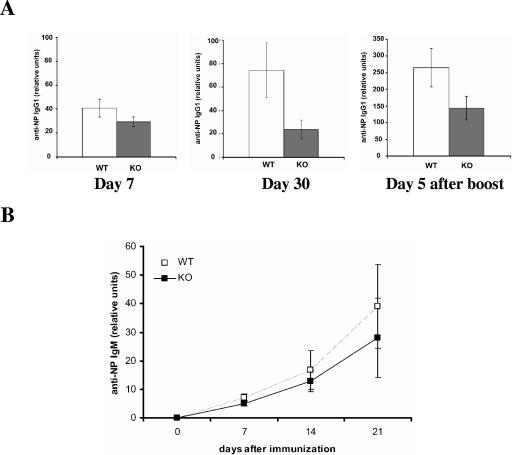
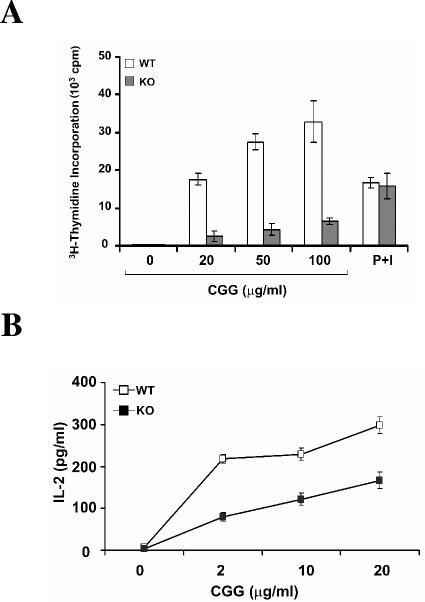
The activation of T lymphocytes plays a central role in the regulation of immune responses (2, 28). To determine whether the reduced T-cell proliferation in HIP-55 knockout mice leads to reduced immune responses, we immunized mice with 4-hydroxy-3-nitrophenylacetyl (NP) coupled to chicken γ globulin (NP-CGG), and antigen-specific antibody production was examined using ELISA. HIP-55 knockout mice showed decreased T-cell-dependent primary immune response as measured by antigen (NP)-specific IgG1 production (Fig. 6A). After rechallenged with NP-CGG, the antigen-specific IgG1 production was also lower in HIP-55 knockout mice (Fig. 6A), which indicates that the secondary immune response was also affected by HIP-55 deficiency. Moreover, the antigen-specific IgM production was also decreased in HIP-55 knockout mice during the primary immune response (Fig. 6B). In addition, when the enlarged lymph nodes were isolated from immunized mice and reactivated with NP-CGG in vitro, HIP-55 knockout cells showed markedly reduced antigen-specific T-cell proliferation and cytokine production as measured by [3H]thymidine incorporation and ELISA assays (Fig. 7A and B). In contrast, phorbol myristate acetate plus ionomycin induced comparable T-cell proliferation in wild-type and knockout mice (Fig. 7A). Thus, HIP-55 knockout mice are defective in T-cell-dependent immune responses as measured by antigen-specific antibody production and T-cell proliferation.
FIG. 6.
Reduced antigen-specific antibody production in HIP-55 knockout mice. (A) Five wild-type (WT) and five HIP-55 knockout (KO) mice were immunized with NP-CGG. Serum was collected at 7 and 30 days (day 0 for secondary response) after the first immunization and 5 days after the second immunization. NP-specific IgG1 production was determined by ELISA and is presented as relative units compared to the standard. Statistical analysis was performed using the Student t test. The data are derived from five mice in each group; the difference is statistically significant (P < 0.02 for day 7, P < 0.01 for day 30 and day 5 after the boost). (B) Anti-NP-specific IgM from immunized mice was measured as described for A.
FIG. 7.
Decreased antigen-specific T-cell proliferation and cytokine production. Wild-type (WT) and HIP-55 knockout (KO) mice were immunized with NP-CGG. Seven days later, the cells from enlarged draining lymph nodes were isolated and restimulated with CGG or phorbol myristate acetate (PMA) plus ionomycin (I) for 64 h, and proliferation was measured using thymidine incorporation assay (A) and interleukin-2 in the supernatant was measured using ELISA (B). Data are representative of three independent experiments. The error bars are standard deviations from replicates of the representative experiment. The difference is statistically significant (P < 0.01).
Decreased JNK/HPK1 activation and LAT/PLCγ1 phosphorylation in HIP-55 knockout T cells.
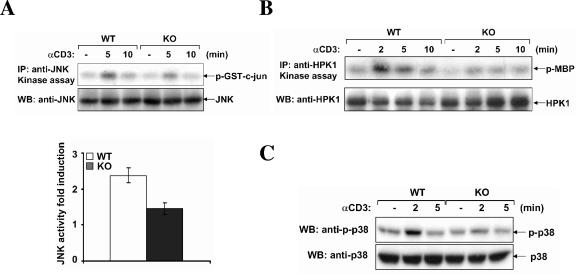
HIP-55 has been shown to be important for JNK and HPK1 activation in TCR signaling (14). Therefore, we examined the TCR-induced activation of HPK1/JNK cascade in HIP-55 knockout T cells. After TCR stimulation, JNK and HPK1 were immunoprecipitated from the cell lysate and in vitro kinase assays were performed using GST-c-Jun or myelin basic protein as a substrate. We found that both JNK and HPK1 activation after TCR stimulation was partially defective in HIP-55 knockout T cells (Fig. 8A and B), which is consistent with previous results (14). We also examined the activation of another mitogen-activated protein kinase, p38, using an antibody specific for phospho-p38 and found that the activation of p38 was also decreased in HIP-55 knockout T cells (Fig. 8C).
FIG. 8.
Decreased activation of HPK1/JNK in HIP-55 knockout T cells. Wild-type (WT) and HIP-55 knockout (KO) T cells were stimulated using an anti-CD3 antibody and in vitro immunocomplex kinase assays were performed to examine JNK (A) and HPK1 (B) kinase activity using GST-c-Jun or myelin basic protein (MBP) as a substrate. The kinase assays were performed three times and the induction of JNK kinase activity at 5 min was quantitated using the Kodak Image Station (lower panel in A). The error bars are standard deviations for the three experiments. The difference is statistically significant (P < 0.01). Western blotting was performed to examine the activation of p38 using an anti-phospho-p38 antibody (C).
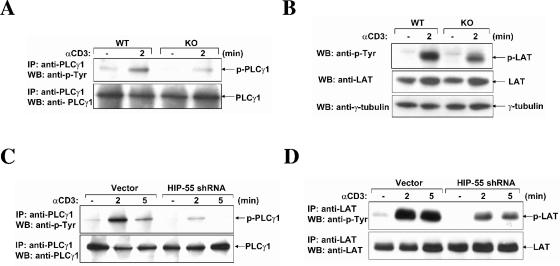
Besides the HPK1 and JNK pathways, the activation (tyrosine phosphorylation) of PLCγ1 and LAT after TCR stimulation was also investigated in HIP-55 knockout T cells. PLCγ1 was immunoprecipitated from an anti-CD3 antibody-treated T cells and the phosphorylation level was examined using an antiphosphotyrosine antibody. We found that the phosphorylation of PLCγ1 was reduced in HIP-55 knockout T cells compared to wild-type T cells (Fig. 9A). Moreover, the inducible phosphorylation of LAT after TCR stimulation was also decreased in HIP-55 knockout T cells (Fig. 9B).
FIG. 9.
Decreased phosphorylation of PLCγ1 and LAT in HIP-55 knockout T cells and HIP-55 shRNA knockdown Jurkat T cells. Wild-type (WT) and HIP-55 knockout (KO) purified splenic T cells were stimulated using an anti-CD3 antibody. (A) The cell lysates were prepared and subjected to immunoprecipitation with an anti-PLCγ1 antibody. Western blot analysis was performed using antiphosphotyrosine and anti-PLCγ1 antibodies. (B) Cell lysates from unstimulated or stimulated T cells of wild-type and knockout mice were subjected to Western blot analysis using antiphosphotyrosine (anti-p-Tyr), anti-LAT, and anti-γ-tubulin antibodies. (C and D) Jurkat T cells were stimulated using an anti-CD3 antibody (OKT3). Cell lysates from vector-transfected and HIP-55 shRNA-transfected Jurkat T cells were prepared and subjected to immunoprecipitation with anti-PLCγ1 (C) and anti-LAT (D) antibodies. Western blot analysis was performed using antiphosphotyrosine, anti-PLCγ1 (C), and anti-LAT (D) antibodies.
To further confirm these results, the tyrosine phosphorylation of LAT and PLCγ1 was examined in HIP-55 knockdown Jurkat T cells using shRNA (14) and similar signaling defects were observed (Fig. 9C and D). Taken together, our results demonstrate that HIP-55 is important for TCR-induced signaling events, including HPK1/JNK activation and LAT/PLCγ1 phosphorylation.
DISCUSSION
Our results have revealed that HIP-55 is an important regulator of TCR signaling and the immune system. In HIP-55 knockout mice, T cells showed reduced proliferation, lower cytokine production, and decreased activation marker up-regulation compared to wild-type T cells after TCR stimulation. The reduced T-cell activation led to the reduced antigen-specific immune responses in HIP-55 knockout mice. The TCR-induced activation of HPK1/JNK and tyrosine phosphorylation of LAT/PLCγ1 were partially defective in HIP-55 knockout T cells.
In a previous report (14), using HIP-55 knockdown Jurkat T cells, we demonstrated that HIP-55 is important for the downstream events, including JNK and HPK1 activation, in TCR signaling. Consistent with our previous results, the HIP-55 knockout T cells showed reduced proliferation and activation. TCR signaling is very important for T-cell development; however, we did not observe any significant developmental defects in the lymphoid organs of the HIP-55 knockout mice, although the defects in TCR signaling in peripheral organs were obvious. It is possible that HIP-55 plays a more critical role in mature T cells than in immature T cells, or perhaps HIP-55 may have different functions at different stages of T-cell development, as shown in several other TCR signaling molecules (30, 31, 33). Moreover, we have not investigated subtler defects in the development of lymphoid organs, which may be affected by HIP-55 deficiency.
Although viable and fertile, HIP-55 knockout mice displayed decreased body weight and increased occurrence of death. Besides its expression and functions in T cells, HIP-55 is a ubiquitously expressed protein (11, 20) and also has known functions in the neuronal system (12). Therefore, the changes we observed in body weight and death incidence may not be related to the immune system. From our observation, most of the deaths of HIP-55 knockout mice occurred in the first 4 weeks after birth. Moreover, the HIP-55 knockout mice showed other unhealthy symptoms, including slightly trembling hind limbs and partial tail paralysis, which are signs of a potential neurological disorder in the HIP-55 knockout mice. It is likely that HIP-55 has important functions in the development of other organs, including the central nervous system, which leads to reduced body weight, a higher frequency of death, and other unhealthy phenotypes in the HIP-55 knockout mice.
The mechanism of how HIP-55 regulates TCR signaling and immune responses is still under investigation. We observed that TCR-induced activation of HPK1/JNK and p38 was partially decreased in HIP-55 knockout T cells, which is consistent with the previous results in HIP-55 shRNA knockdown Jurkat T cells (14). Moreover, the phosphorylation of LAT and PLCγ1 was also decreased in HIP-55 knockout T cells. The decreased activation of TCR signaling events may contribute to the T-cell proliferation and activation defects we observed in HIP-55 knockout mice.
HIP-55 constitutively interacts with HPK1 (11, 22), which binds to several adaptor proteins (4) in TCR signaling complexes, including LAT (23), SLP-76 (29), Gads/Grap2 (25, 26), Grb2 (3, 23), and Crk/CrkL (24). It is possible that HIP-55 may regulate the activation of TCR signaling complexes through HPK1 binding. After TCR stimulation, HIP-55 translocates into lipid rafts (14, 22). As an actin-binding protein, HIP-55 may also potentially affect the immune synapse formation and TCR signaling by regulating the cytoskeleton reorganization.
HIP-55 inducibly interacts with and is phosphorylated by upstream kinase ZAP-70 in TCR signaling (14). Since ZAP-70 is critical for TCR signaling and T-cell functions (10, 32), it is also possible that HIP-55 may function in regulating ZAP-70 activation by modulating the recruitment of ZAP-70 to the TCR complex through cytoskeleton reorganization in TCR signaling. There is evidence indicating that HIP-55 negatively regulates TCR signaling by promoting down-regulation of TCR expression in Jurkat T cells using an overexpression system (22). However, we did not observe any significant increase of TCR expression using HIP-55 knockout primary T cells or shRNA knockdown Jurkat T cells. In contrast, we demonstrated that both CD3ɛ and TCRβ were slightly decreased in HIP-55 knockout T cells before and after TCR stimulation. This slightly accelerated TCR internalization may partially contribute to decreased signaling and other downstream events. The genetic evidence we provided using knockout mice and shRNA knockdown T cells indicates that HIP-55 positively regulates T-cell proliferation and activation.
Besides its role in TCR signaling, it is likely that HIP-55 also has regulatory functions in other signaling pathways involved in antigen-presenting cell functions. It is known that HIP-55 is also involved in B-cell receptor signaling (20), and Syk shares similar regulatory mechanisms with ZAP-70 (21); therefore, it is possible that HIP-55 may also interact with Syk, which affects B cells, or other antigen-presenting cells. It would be worthwhile to study the functions of HIP-55 in different cell types of the immune system.
Acknowledgments
We thank W. Wang and M. Stamnes for generous gifts, members of the Tan laboratory for helpful discussions and critical reading of the manuscript, and D. Guzman for secretarial assistance.
This work was supported by National Institutes of Health grants RO1-AI042532 and RO1-CA87076.
REFERENCES
- 1.Abraham, R. T., and A. Weiss. 2004. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 4:301-308. [DOI] [PubMed] [Google Scholar]
- 2.Acuto, O., and D. Cantrell. 2000. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18:165-184. [DOI] [PubMed] [Google Scholar]
- 3.Anafi, M., F. Kiefer, G. D. Gish, G. Mbamalu, N. N. Iscove, and T. Pawson. 1997. SH2/SH3 adaptor proteins can link tyrosine kinases to a Ste20-related protein kinase, HPK1. J. Biol. Chem. 272:27804-27811. [DOI] [PubMed] [Google Scholar]
- 4.Boomer, J. S., and T.-H. Tan. 2005. Functional interactions of HPK1 with adaptor proteins. J. Cell. Biochem. 95:34-44. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos, M. C., C. Munoz, M. C. Montoya, E. Lara-Pezzi, M. Lopez-Cabrera, and M. O. de Landazuri. 1997. Expression of the leukocyte early activation antigen CD69 is regulated by the transcription factor AP-1. J. Immunol. 159:5463-5473. [PubMed] [Google Scholar]
- 6.Chen, Y.-R., R. Kori, B. John, and T.-H. Tan. 2001. Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis. Biochem. Biophys. Res. Commun. 288:981-989. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y.-R., C. F. Meyer, and T.-H. Tan. 1996. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J. Biol. Chem. 271:631-634. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y.-R., X. Wang, D. Templeton, R. J. Davis, and T.-H. Tan. 1996. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271:31929-31936. [DOI] [PubMed] [Google Scholar]
- 9.D'Ambrosio, D., D. A. Cantrell, L. Frati, A. Santoni, and R. Testi. 1994. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 24:616-620. [DOI] [PubMed] [Google Scholar]
- 10.Elder, M. E. 1998. ZAP-70 and defects of T-cell receptor signaling. Semin. Hematol. 35:310-320. [PubMed] [Google Scholar]
- 11.Ensenat, D., Z. Yao, X. S. Wang, R. Kori, G. Zhou, S. C. Lee, and T.-H. Tan. 1999. A novel src homology 3 domain-containing adaptor protein, HIP-55, that interacts with hematopoietic progenitor kinase 1. J. Biol. Chem. 274:33945-33950. [DOI] [PubMed] [Google Scholar]
- 12.Fenster, S. D., M. M. Kessels, B. Qualmann, W. J. Chung, J. Nash, E. D. Gundelfinger, and C. C. Garner. 2003. Interactions between Piccolo and the actin/dynamin-binding protein Abp1 link vesicle endocytosis to presynaptic active zones. J. Biol. Chem. 278:20268-20277. [DOI] [PubMed] [Google Scholar]
- 13.Fucini, R. V., J. L. Chen, C. Sharma, M. M. Kessels, and M. Stamnes. 2002. Golgi vesicle proteins are linked to the assembly of an actin complex defined by mAbp1. Mol. Biol. Cell 13:621-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, J., R. Kori, J.-W. Shui, Y.-R. Chen, Z. Yao, and T.-H. Tan. 2003. The SH3 domain-containing adaptor HIP-55 mediates c-Jun N-terminal kinase activation in T cell receptor signaling. J. Biol. Chem. 278:52195-52202. [DOI] [PubMed] [Google Scholar]
- 15.Hu, M.-C., W. R. Qiu, X. Wang, C. F. Meyer, and T.-H. Tan. 1996. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 10:2251-2264. [DOI] [PubMed] [Google Scholar]
- 16.Janssen, E., and W. Zhang. 2003. Adaptor proteins in lymphocyte activation. Curr. Opin. Immunol. 15:269-276. [DOI] [PubMed] [Google Scholar]
- 17.Kessels, M. M., A. E. Engqvist-Goldstein, and D. G. Drubin. 2000. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), an Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol. Biol. Cell 11:393-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessels, M. M., A. E. Engqvist-Goldstein, D. G. Drubin, and B. Qualmann. 2001. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol. 153:351-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koretzky, G. A., and P. S. Myung. 2001. Positive and negative regulation of T-cell activation by adaptor proteins. Nat. Rev. Immunol. 1:95-107. [DOI] [PubMed] [Google Scholar]
- 20.Larbolette, O., B. Wollscheid, J. Schweikert, P. J. Nielsen, and J. Wienands. 1999. SH3P7 is a cytoskeleton adapter protein and is coupled to signal transduction from lymphocyte antigen receptors. Mol. Cell. Biol. 19:1539-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latour, S., and A. Veillette. 2001. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 13:299-306. [DOI] [PubMed] [Google Scholar]
- 22.Le Bras, S., I. Foucault, A. Foussat, C. Brignone, O. Acuto, and M. Deckert. 2004. Recruitment of the actin-binding protein HIP-55 to the immunological synapse regulates T cell receptor signaling and endocytosis. J. Biol. Chem. 279:15550-15560. [DOI] [PubMed] [Google Scholar]
- 23.Ling, P., C. F. Meyer, L. P. Redmond, J.-W. Shui, B. Davis, R. R. Rich, M.-C. Hu, R. L. Wange, and T.-H. Tan. 2001. Involvement of hematopoietic progenitor kinase 1 in T cell receptor signaling. J. Biol. Chem. 276:18908-18914. [DOI] [PubMed] [Google Scholar]
- 24.Ling, P., Z. Yao, C. F. Meyer, X. S. Wang, W. Oehrl, S. M. Feller, and T.-H. Tan. 1999. Interaction of hematopoietic progenitor kinase 1 with adapter proteins Crk and CrkL leads to synergistic activation of c-Jun N-terminal kinase. Mol. Cell. Biol. 19:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou, J., F. Kiefer, A. Dang, A. Hashimoto, M. H. Cobb, T. Kurosaki, and A. Weiss. 2000. HPK1 is activated by lymphocyte antigen receptors and negatively regulates AP-1. Immunity 12:399-408. [DOI] [PubMed] [Google Scholar]
- 26.Ma, W., C. Xia, P. Ling, M. Qiu, Y. Luo, T.-H. Tan, and M. Liu. 2001. Leukocyte-specific adaptor protein Grap2 interacts with hematopoietic progenitor kinase 1 (HPK1) to activate JNK signaling pathway in T lymphocytes. Oncogene 20:1703-1714. [DOI] [PubMed] [Google Scholar]
- 27.Mise-Omata, S., B. Montagne, M. Deckert, J. Wienands, and O. Acuto. 2003. Mammalian actin binding protein 1 is essential for endocytosis but not lamellipodia formation: functional analysis by RNA interference. Biochem. Biophys. Res. Commun. 301:704-710. [DOI] [PubMed] [Google Scholar]
- 28.Samelson, L. E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371-394. [DOI] [PubMed] [Google Scholar]
- 29.Sauer, K., J. Liou, S. B. Singh, D. Yablonski, A. Weiss, and R. M. Perlmutter. 2001. Hematopoietic progenitor kinase 1 associates physically and functionally with the adaptor proteins B cell linker protein and SLP-76 in lymphocytes. J. Biol. Chem. 276:45207-45216. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, E. M., J. Debnath, G. Yap, D. McVicar, X. C. Liao, D. R. Littman, A. Sher, H. E. Varmus, M. J. Lenardo, and P. L. Schwartzberg. 1999. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284:638-641. [DOI] [PubMed] [Google Scholar]
- 31.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. protein kinase C-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 32.Williams, B. L., K. L. Schreiber, W. Zhang, R. L. Wange, L. E. Samelson, P. J. Leibson, and R. T. Abraham. 1998. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18:1388-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong, X. P., E. A. Hainey, B. A. Olenchock, M. S. Jordan, J. S. Maltzman, K. E. Nichols, H. Shen, and G. A. Koretzky. 2003. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 4:882-890. [DOI] [PubMed] [Google Scholar]