Abstract
Homologous recombination (HR) requires nuclease activities at multiple steps, but the contribution of individual nucleases to the processing of double-strand DNA ends at different stages of HR has not been clearly defined. We used chicken DT40 cells to investigate the role of flap endonuclease 1 (Fen-1) in HR. FEN-1-deficient cells exhibited a significant decrease in the efficiency of immunoglobulin gene conversion while being proficient in recombination between sister chromatids, suggesting that Fen-1 may play a role in HR between sequences of considerable divergence. To clarify whether sequence divergence at DNA ends is truly the reason for the observed HR defect in FEN-1−/− cells we inserted a unique I-SceI restriction site in the genome and tested various donor and recipient HR substrates. We found that the efficiency of HR-mediated DNA repair was indeed greatly diminished when divergent sequences were present at the DNA break site. We conclude that Fen-1 eliminates heterologous sequences at DNA damage site and facilitates DNA repair by HR.
Homologous recombination (HR) plays a critical role in genome maintenance by repairing double-strand breaks (DSBs) induced by exogenous agents or occurring during DNA replication. In chicken B lymphocyte precursors, HR mediates the diversification of the immunoglobulin (Ig) variable region, a process called gene conversion (7, 31, 33, 45). HR is also essential when transfected DNA is integrated in the genome at specific sites of homology during gene targeting.
HR is initiated by DNA damage, including DSBs and single-strand breaks. It involves interactions between damaged DNA and intact homologous sequences and results in the transfer of genetic information from the intact donor to the damaged recipient. HR-dependent DSB repair has been well studied in the budding yeast by use of the HO restriction enzyme. The initial step involves processing of DNA ends to produce a 3′ single-strand overhang, which is covered by Rad51. The resulting nucleoprotein filament is responsible for homology search, homologous pairing, and strand invasion (D loop formation) followed by DNA synthesis from the 3′ end of the invading strand (46). This HR-dependent repair of HO-induced DSBs requires a number of nucleases (13). Firstly, the 3′ overhang formation at DSBs appears to be carried out by Mre11 and Exo1 and perhaps by other unknown nucleases in mitotic cells (12, 20, 24, 40, 41). Secondly, nonhomologous tails from the 3′ overhang including the HO site should be eliminated. These overhangs interfere with subsequent steps of HR by destabilizing the D loop and precluding 3′-OH-end extensions (26). In higher eukaryotic cells, the end processing of DNA during the initial step of HR is more controversial. For example, mre11-deficient DT40 cells exhibit normal kinetics of Rad51 focus formation after irradiation (IR) (48), indicating that induced DSBs are processed normally in the absence of Mre11. Likewise, exo1-deficient mouse embryonic stem cells show defective mismatch repair but normal HR capability (44).
Fen-1 is a structure-specific nuclease that cleaves 5′ flaps of the branched DNA structures and possesses double-strand-specific 5′-to-3′ exonuclease activity (14, 27). Recently Zheng et al. (51) reported that Fen-1 cleaves DNA bubble structures by 5′ and 3′ incision in vitro. The endonuclease activity of Fen-1 is required for processing the 5′ ends of Okazaki fragments in lagging strand DNA synthesis (42, 43). Fen-1 also contributes to base excision repair (BER) by removing 5′ flap structures formed during gap-filling DNA synthesis (28). This notion is supported by the phenotype of FEN-1-deficient DT40 cells, which are hypersensitive to killing by alkylating agents such as methylmethane sulfonate and hydroxyperoxide (22). Consistent with the important role for Fen-1 in DNA replication and BER, FEN-1−/− mice are lethal during early embryogenesis (19), and even mice heterozygous for FEN-1 display a high incidence of tumorigenesis, presumably due to genome instability (18).
Although mammalian mutants deficient in FEN-1 are not viable, FEN-1−/− DT40 cells are able to proliferate with slightly elongated cell cycle time (22). Thus, FEN-1−/− DT40 cells provide a novel opportunity to analyze in vivo functions of vertebrate Fen-1. DT40 cells are useful for comprehensive analysis of a variety of HR reactions, because a number of phenotypic assays have been developed (15, 31, 50). These assays include the measurement of the rate of Ig gene conversion, sister chromatid exchange (SCE), gene targeting, repair of DSBs created in artificial constructs, and repair of DSBs induced by ionizing radiation (IR) at the late S to G2 phase (1, 2, 4, 32, 34, 35, 36, 37). Using these assays we found in this study that Fen-1 is required for HR between homologous sequences with nonhomologous tails at the DNA break ends while it is dispensable for HR between perfect homologies.
MATERIALS AND METHODS
Plasmid constructs.
Two XPG disruption constructs, XPG-hisD and XPG-bsr, were generated from genomic PCR products combined with hisD- and bsr-selection marker cassettes (see Fig. S2 in the supplemental material). Genomic DNA sequences were amplified using the primers 5′-TCTGATACATGAACTGACAGATAAGCACAG-3′ and 5′-CGGGATCCGTCTGCTAAAGTAACTCACCACCACAAGC-3′ (for the left arm of the disruption construct) and 5′-CTCGGATCCTTTTCCCAGTACCAGCTTAGGGGTTTGC-3′ and 5′-GAGGGTACCTGAAGCATTTCCTGCTCAGCAGAAAGGTC-3′ (for the right arm of the disruption construct). Amplified PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen). The 2.1-kb NotI-BamHI fragment from the left arm and the 2.5-kb BamHI-KpnI fragment from the right arm were cloned into NotI and KpnI sites of pBluescript KS, respectively. The BamHI site between the two arms was used to clone marker gene cassettes. For the probe used in Southern blot analysis, the genomic DNA was amplified using the primers 5′-GCTACTTCTGTAACAGGACAAATGTTCTTG-3′ and 5′-ATAACCAAACATCACTATCATCAGTGATTG-3′ and was digested with EcoRV to obtain a 1-kb fragment. The expression vector pCR3-loxP-XPG/IRES-EGFP-loxP, in which the XPG and enhanced green fluorescent protein (EGFP) genes are flanked by two loxP sequences, was constructed by inserting an XPG SalI-BamHI cDNA fragment between the SalI and BamHI sites of pCR3-loxP-MCS-loxP (49). Gene targeting substrates and heterologous sequences inserted into S2neo reporter gene were amplified with primers listed elsewhere (see Table S1 in the supplemental material). Expression vectors of chicken FEN-1 and nuclease-dead mutant of FEN-1 were given by Keizo Tano (Research Reactor Institute, Kyoto University, Osaka, Japan).
Cell culture, DNA transfection, and γ irradiation.
Cells were cultured in RPMI 1640 supplemented with 10−5 M β-mercaptoethanol, 10% fetal calf serum, and 1% chicken serum (Sigma, St Louis, MO) at 39.5°C. Methods of DNA transfection for producing stable transfectants and genotoxic treatments were as described previously (34). Cell synchronization was achieved by elutriation as described previously (34). 137Cs (Gammacell 40, Nordion, Kanata, Ontario, Canada) (0.02 Gy/s) was used for γ irradiation.
Measurement of SCE levels.
SCE levels were measured as described previously (49).
Analysis of Ig gene conversion.
FEN-1−/− cells were established from CL18, a subclone of DT40 cells that is negative for surface IgM (sIgM) (3, 22). We confirmed that FEN-1−/− cells retained the same frameshift mutation as do wild-type CL18 cells by sequencing the Ig Vλ region. The rate of Ig gene conversion was assessed by measuring the gain of sIgM expression during a 3-week period as described previously (3).
I-SceI-induced gene conversion and gene targeting.
A total of 107 cells were suspended in 0.1 ml Nucleofector Solution T (Amaxa biosystems) and electroporated using an Amaxa system (Amaxa biosystems) at program B-23. For the gene conversion assay, 5 μg of circular I-SceI expression vector (pcBASce) with or without nuclease expression vector was transfected into the cells. For the gene targeting assay, 2 μg of substrate DNA and 4 μg of pcBASce with or without nuclease expression vector was transfected. pBluescript II KS+ was used as a negative control. At 24 h after electroporation, the number of live cells were counted by fluorescence-activated cell sorting (FACS) and the cells were transferred to 96-well cluster trays with or without 2.0 mg of G418 per ml. Cells were grown for 7 to 10 days, and HR frequencies were calculated by the following equation: HR frequency (colonies/cell) = number of G418-resistant colonies/(plating efficiency of transfected cells in the absence of G418 × number of live cells determined by FACS 24 h after electroporation).
Measurement of targeted integration frequencies.
To analyze the targeted integration events at the Ovalbumin (4), RAD54 (2), and β-ACTIN loci, each disruption construct was transfected into cells, and Southern blot analysis was performed following selection of clones against appropriate antibiotics. For the CENP-H locus, a CENP-H-EGFP knock-in construct (10) was used, and the targeted events were scored by FACS analysis.
Nucleotide sequence accession number.
The chicken XPG cDNA sequences have been submitted to the GenBank database under accession number AB063480.
RESULTS
Reduced kinetics of the Ig gene conversion in FEN-1−/− cells.
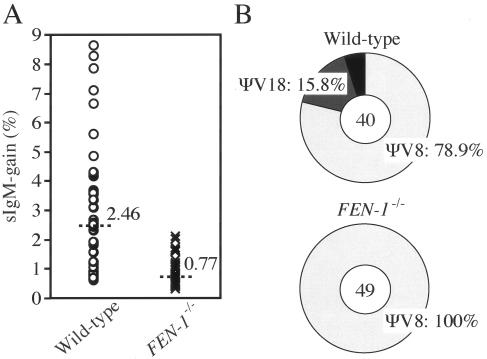
We have screened nucleases that are required for HR by analyzing the rate of Ig gene conversion in nuclease gene-disrupted DT40 clones. In this assay, intragenic gene conversion between two diverged homologous sequences, i.e., donor pseudo-V (ΨV) and recipient VJλ segments, allows determination of gene conversion events as well as the identification of aberrant events (29). To assess the kinetics of Ig gene conversion, we measured the gain of surface IgM (sIgM) expression, which may reflect elimination of a given frameshift mutation at the recipient VJλ by superimposed gene conversion events (3, 30). Interestingly, FEN-1−/− cells exhibited a 3.2-fold reduction of sIgM gain (Fig. 1A), suggesting a role for Fen-1 in Ig gene conversion. In contrast, gene conversion was not impaired in cells deficient in XPG (data not shown), which belongs to the same nuclease group as Fen-1 (21). Since a defect in Ig gene conversion is often accompanied by the alteration of the usage of donor ΨV segments, we determined nucleotide sequences of the Ig Vλ segment in the cells that acquired sIgM expression. We found that the usage of ΨV segments was different between wild-type and FEN-1−/− cells, while no significant alternation was found in their gene conversion tract length. The ΨV8 segment was used in 78.9% of the gene conversion events in wild-type cells, while all analyzed 49 gene conversion events exclusively involved ΨV8 in FEN-1−/− cells (Fig. 1B). The ΨV8 donor segment shares the highest homology with the VJλ recipient segment among the ΨV segments (3). Thus, we conclude that deletion of FEN-1 reduced the frequency of Ig gene conversion involving ΨV8 by 2.5-fold and completely abolished Ig gene conversion with more-diverged ΨV donor segments. These observations imply that Fen-1 may be involved in HR, particularly between diverged homologous sequences.
FIG. 1.
Reduced frequency of Ig gene conversion in FEN-1−/− cells. (A) Fluctuation analysis of appearance of sIgM-gain revertants. The abundance of sIgM-gain revertants was determined in parallel cultures derived from sIgM− single cells after 3-week clonal expansion; median percentages are noted above each data set and are indicated by the line. (B) Preference of pseudo-V gene usage as a donor for Ig gene conversion among isolated sIgM-gain populations. The total number of Vλ sequences analyzed is indicated in the center of the charts.
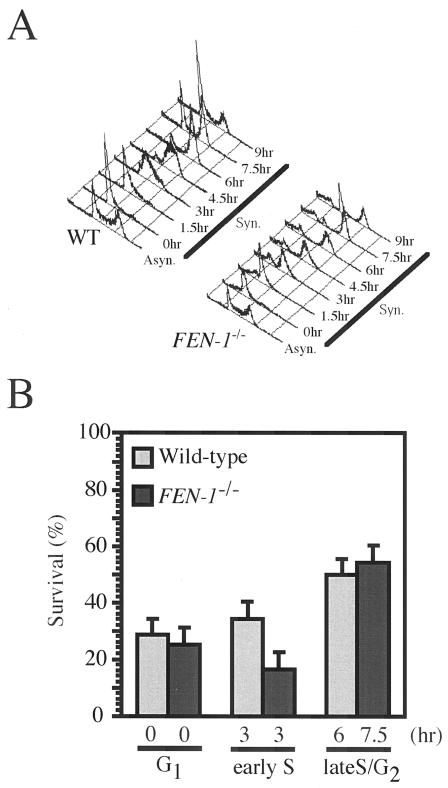
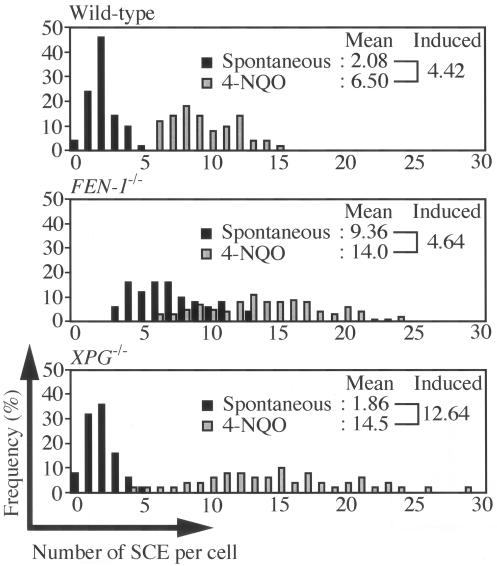
The reduced kinetics of Ig gene conversion in FEN-1−/− cells led us to perform other phenotypic assays of HR reactions. We previously showed that HR-deficient clones such as RAD54−/− cells exhibit elevated IR sensitivity specifically in the late S to G2 phase, when sister chromatids are available to provide exactly matching repair templates for HR (2, 34). We analyzed the sensitivity of synchronized populations of cells to killing by γ radiation using a colony formation assay. Unlike RAD54−/− cells, FEN-1−/− cells showed elevated IR sensitivity in the early S phase but not in the late S to G2 phase (Fig. 2B), indicating that Fen-1 is dispensable for HR between sister chromatids. We next determined the level of microscopically visible SCE events, which display gene conversion associated with crossover between two sister chromatids (32). Consistent with the hyper-recombination phenotype of the budding yeast rad27Δ strain (39), FEN-1−/− DT40 cells showed about 4.5-fold-higher spontaneous SCE levels than did wild-type cells (Fig. 3). When we induced SCE by exposing the cells to 4-nitroquinoline 1-oxide (4-NQO), which mimics UV damage (9), the levels of induced SCE were very similar between wild-type and FEN-1−/− cells (Fig. 3). These data suggest that Fen-1 is not required for HR between identical sister chromatids.
FIG. 2.
γ-Ray sensitivity of cells at the G1, early S, and late S/G2 phases. (A) Cells of the indicated genotypes were synchronized at the G1 phase with elutriation and released into culture at 0 h. WT, wild type. (B) Wild-type or FEN-1−/− cells were exposed to 2-Gy γ-rays at the indicated cell cycle phase. The number of colonies which appeared after irradiation was divided by that of nonirradiated controls; results are shown as % survival.
FIG. 3.
The level of induced SCE was indistinguishable between wild-type and FEN-1−/− cells. The histogram indicates the number of SCE per cell in the wild-type, FEN-1−/−, and XPG−/− cells. The values of induced SCE were calculated by subtracting the mean value of nontreated cells from that of 4NQO-treated cells; results are shown at the top of each panel. Black and gray bars indicate spontaneous SCE as well as SCE induced by 0.2 ng/ml 4NQO treatment, respectively.
Defective I-SceI restriction enzyme-induced gene conversion in FEN-1−/− cells.
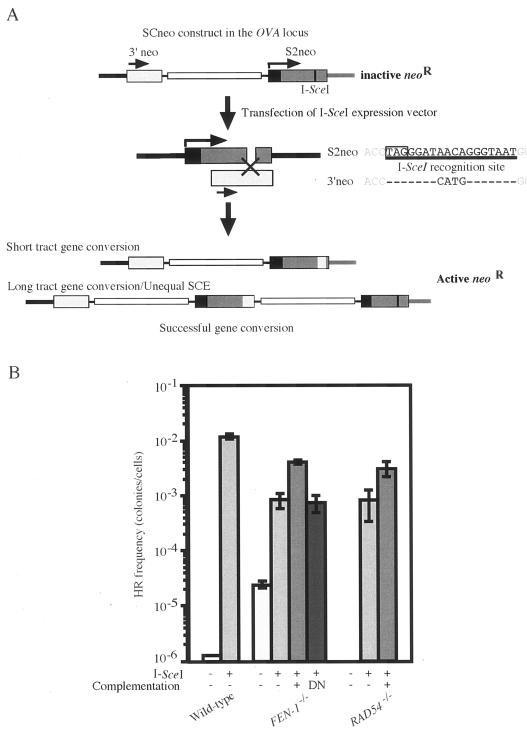
It is believed that Ig gene conversion is initiated by single-strand damage, which is generated as the consequence of AID deaminase-dependent formation of uracil at the Ig V gene (1, 6). To test whether Fen-1 is required not only for Ig gene conversion that is induced by single-strand DNA damage but also for DSB-induced HR, we used an artificial gene conversion substrate DNA, SCneo (16). In the SCneo construct, two mutated neomycin-resistance genes (neoR) that are complementary to each other are localized in tandem. The recipient neoR coding region is disrupted by the 18-bp I-SceI cleavage site including a stop codon (S2neo; Fig. 4A), while the other promoterless intact neoR gene serves as a genetic donor for recombinational repair of the I-SceI-induced DSB. To accurately compare data of wild-type and FEN-1−/− cells, we integrated the SCneo substrate at the Ovalbumin locus in each genotype (11). DSBs induced by transient expression of I-SceI are repaired by gene conversion either from the upstream donor homologous sequences (intragenic gene conversion) or the other sister chromatid (unequal sister recombination) (16). Of note, the 18 nucleotides comprising the I-SceI site in the SCneo recipient sequence should be eliminated during gene conversion-dependent DSB repair to reconstitute a functional neoR gene in the cells. Accordingly, we found that HR-mediated DSB-repair induced by transient expression of I-SceI was reduced more than 10-fold in the FEN-1−/− cells while there was no significant decrease in colony number in XPG−/− cells (Fig. 4B and data not shown). Notably, the reduction in I-SceI gene conversion efficiency was partially reversed by transfection of chicken FEN-1 cDNA into FEN-1−/− cells but not by a nuclease-dead mutant of chicken FEN-1 (Fig. 4B). Overexpression of chicken FEN-1 in wild-type cells enhanced I-SceI-induced gene conversion by 2.87-fold (see Fig. S1 in the supplemental material). These data indicate that Fen-1 is required for efficient DSB-induced gene conversion (Fig. 4B) as well as single-strand-damage-induced Ig gene conversion (Fig. 1), and the nuclease activity of Fen-1 is important for this process.
FIG. 4.
The reduction of I-SceI-induced gene conversion in FEN-1−/− cells. (A) Experimental method of measuring the frequency of gene conversion by counting G418-resistant colonies. The expression vector encoding I-SceI is introduced into cells carrying SCneo in the Ovalbumin locus. Black and gray boxes in S2neo represent the 5′ untranslated and coding regions of the neoR gene, respectively. The figure is not drawn to scale. Successful gene conversion would reconstitute functional neoR gene. (B) The recombination frequency in the SCneo reporter construct in each genotype is shown as the number of G418-resistant colonies derived from 107 cells transfected with the indicated plasmid. Complementation denotes cotransfection of the I-SceI expression plasmid with expression vector of the disrupted gene in the indicated transfected cells. DN, an expression plasmid for nuclease-dead mutant of chicken FEN-1. The experiments were done more than four times.
Fen-1 is involved in elimination of heterologous sequences at the I-SceI cleavage site.
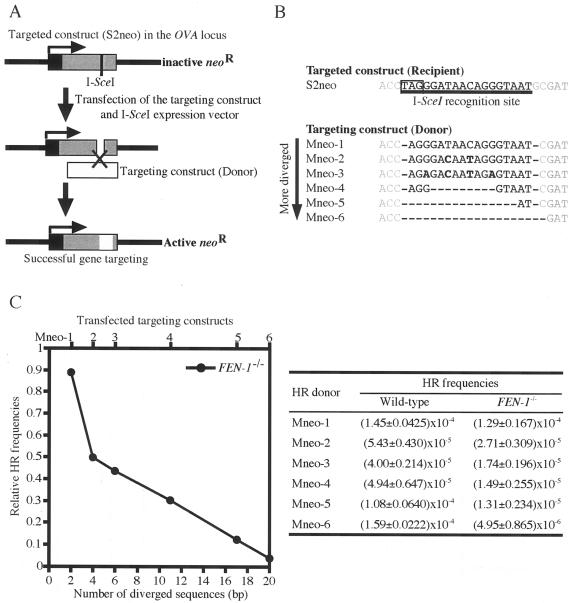
To investigate whether the sequence divergence between donor and recipient was indeed responsible for the reduction of HR frequency in the absence of Fen-1, we examined I-SceI-induced gene targeting as previously studied (8). Wild-type or FEN-1−/− cells carrying S2neo targeted sequence at the Ovalbumin locus were transiently transfected with the donor construct and the I-SceI expression vector (Fig. 5A). We used a series of donor constructs carrying different degrees of sequence divergence at I-SceI site (Fig. 5B). Among these modified targeting constructs, Mneo-1 had the smallest sequence divergence from the intact I-SceI site, differing only at two residues. When successfully targeted, these modified sequences will replace the stop codon in I-SceI site and be translated into four extra (Mneo-1, Mneo-2, Mneo-3), one extra (Mneo-4), one fewer (Mneo-5), or two fewer (Mneo-6) amino acid residue(s) in the neoR gene product compared to the wild-type neoR gene. Using this series of targeting constructs we found that Mneo-1 gave a similar number of colonies when transfected into FEN-1−/− cells and wild-type cells carrying S2neo. Conversely, the gene targeting efficiency in FEN-1−/− cells, compared to wild-type cells, decreased with the extent of heterologous sequence at the site of DNA breaks (Fig. 5C). These data support our notion that Fen-1 facilitates HR by eliminating heterologous sequences at the I-SceI site.
FIG. 5.
Diverged short sequences block effective recombination in FEN-1−/− cells. (A) Experimental method of measuring the frequency of gene targeting by counting G418-resistant colonies. The expression vector encoding I-SceI is introduced together with WTneo (white box) into cells carrying S2neo in the Ovalbumin locus. Black and gray boxes represent the 5′ untranslated and coding regions of the neoR gene, respectively. The figure is not drawn to scale. Successful gene targeting would reconstitute a functional neoR gene. (B) Base sequence alignment around the I-SceI site in a series of targeting constructs (Mneo-1 to Mneo-6). Mneo-1 donor contains sequences that are two nucleotides shorter (shown by hyphen) than the corresponding sequences of S2neo recipient. Bold characters show inserted point mutations. Boxed characters show a stop codon. (C) The gene-targeting frequency of targeting constructs in FEN-1−/− cells. The indicated targeting constructs (shown at top) were transfected into cells carrying S2neo. The number of diverged sequences (shown at bottom) includes inserted point mutations and missing sequences in the targeting constructs. Relative HR frequencies on the y axis were calculated by dividing the HR frequency of FEN-1−/− cells which appeared after G418 selection by that of FEN-1+/+ cells.
DISCUSSION
In the present study, we provide the first evidence that Fen-1 is involved in HR reactions in higher eukaryotic cells. Interestingly, only a subset of HR events is affected by loss of Fen-1. Clearly FEN-1−/− cells are not IR sensitive during the G2 phase of the cell cycle and display wild-type levels of induced SCE. However, the HR reaction appears to be affected in FEN-1−/− cells when sequence variation between recipient and donor occurs. Thus, Ig gene conversion, which is a good example of HR between variable sequences, is significantly reduced in FEN-1−/− cells. Moreover, the mutant cells exclusively use the ΨV8 donor segment, which shares the highest homology with VJλ recipient segment.
Lastly, our S2neo reporter assay clearly points to a role of Fen-1 in removing nonhomologous sequences during the HR reaction. In these experiments, which were thoroughly controlled for variations in plating efficiency and sensitivity to the endonuclease expression, gene targeting efficiency decreased with the extent of sequence divergence only in the FEN-1−/− mutants but not in wild-type and XPG−/− cells. One could argue that the HR frequency could be affected by the enzymatic activities of reconstituted neoR gene products. The use of various Mneo constructs may cause variation in the number of G418-resistant colonies, since the introduction of sequence divergence at the I-SceI site results in the changes of amino acids in neomycin phosphotransferase. This was not the case, since each Mneo construct showed similar HR frequencies in wild-type cells (Fig. 5C). Another point of concern is the possibility that HR frequency can be affected by nonhomologous end-joining (NHEJ) activity. As long as DSB ends are ligated precisely by NHEJ, these sites are subject to perpetual digestion due to the constitutive expression of I-SceI restriction enzyme. Either HR or imprecise ligation by NHEJ eliminates the I-SceI site and terminates the reaction. The latter case leads to the apparent decrease in HR frequency due to the improperly reconstituted neoR gene. We excluded this possibility by a plasmid religation assay (37), in which we observed a normal NHEJ activity in FEN-1−/− cells (data not shown). Taken together, these data indicate that the HR defect in FEN-1−/− cells clearly depends on the divergence between recipient and donor sequences. Fen-1 is dispensable for the HR reactions that occur between identical or highly homologous sequences such as two sister chromatids or ΨV8 segment but is required for the recombination between DNAs that have short nonhomologous sequences at the ends. Our findings are also consistent with previous yeast genetics results (25). Fen-1 is localized together with PCNA close to the chromatin at pachytene when meiotic recombination between homologous chromosomes occurs (17). It is tempting to speculate that Fen-1 may also facilitate recombination by removal of heterologous sequences between maternal and parental chromosomes during meiosis in higher eukaryotes.
We observed an elevated level of spontaneous SCE in FEN-1−/− cells, which apparently argues against the involvement of Fen-1 in HR. However, this finding is consistent with results obtained from phenotypic analysis of the yeast rad27Δ strain (39), where the frequency of mitotic crossover is increased and is thought to be a consequence of defective lagging strand DNA synthesis (42, 44). The resulting defect in DNA replication appears to be replaced by HR-mediated repair, because budding yeast mutants deficient in both rad27 and RAD52 epistasis groups are synthetically lethal (5, 38). Likewise, our observation of increased spontaneous SCE in FEN-1−/− cells may reflect enhanced HR-dependent repair due to defective processing of Okazaki fragments and impaired BER. Accordingly, we observed increased IR sensitivity during early S phase, which could be a consequence of defective BER and subsequent replication blocking in FEN-1−/− cells.
How Fen-1 eliminates nonhomology from DNA ends remains elusive. It has been shown in vitro that Fen-1 possesses 5′-to-3′ exonulease as well as structure-specific endonuclease activity. We speculate on three possibilities for the action of Fen-1 based on the biochemical evidence presented so far by others. First, Fen-1 may extend the 3′ overhang with its exonucleolytic activity until the identical sequence is exposed, so that the pairing can occur between the substrate and homologous template DNA. Secondly, the endonuclease activity of Fen-1 may eliminate nonhomologous 5′ flap structures at D-loops after the invasion of 5′ overhang into duplex DNA. This idea is consistent with biochemical evidence that Fen-1 cleaves the 5′ and not the 3′ flap structure of DNA and with the observation that Rad51 can form filaments on 5′ single-stranded DNA and perform strand exchange (23). The third of the possibilities relies on the recent observations made by Zheng et al. (51), in which DNA bubble structures are cleaved by Fen-1 at single- and double-stranded DNA junctions on both ends. This suggests that, under some circumstances, Fen-1 could cleave the 3′ flap structure, which is generated after strand invasion by 3′ overhang. The first and third possibilities are not mutually exclusive and may in fact complement each other. Accumulating evidence has suggested a critical role of Fen-1 for processing DSB ends in the course of HR (25) and NHEJ (47) in yeast. This study sheds light on a previously unknown function of Fen-1 in higher eukaryotic cells: the elimination of imperfectly matched sequences from DSB ends for subsequent HR-mediated DSB repair. Interestingly, Fen-1 also contributes to conventional gene targeting at three different loci (see Table S2 in the supplemental material). Although the role for Fen-1 in this situation is unclear, it is tempting to speculate that Fen-1 functions in removing the heterologous sequences as it does in I-SceI-induced gene targeting. Since overexpression of Fen-1 alone enhanced I-SceI-induced gene targeting (see Fig. S1 in the supplemental material), the challenge for the future will be to seek for the way to improve gene targeting efficiency in vertebrate cells using Fen-1 and to understand how Fen-1 works in conventional gene targeting.
Supplementary Material
Acknowledgments
We thank K. Tano for expression vectors of chicken FEN-1 and nuclease-dead mutant of FEN-1 and Y. Kitawaki, Y. Sato, S. Okajima, and R. Ohta for their technical assistance.
Financial support was provided in part by a grant of Core Research for Evolutional Science and Technology (CREST) from Japan Science and Technology Corporation, by a Center of Excellence (COE) grant for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government, and by grants from The Uehara Memorial Foundation and The Naito Foundation.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arakawa, H., J. Hauschild, and J. M. Buerstedde. 2002. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295:1301-1306. [DOI] [PubMed] [Google Scholar]
- 2.Bezzubova, O., A. Silbergleit, Y. Yamaguchi-Iwai, S. Takeda, and J. M. Buerstedde. 1997. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell 89:185-193. [DOI] [PubMed] [Google Scholar]
- 3.Buerstedde, J. M., C. A. Reynaud, E. H. Humphries, W. Olson, D. L. Ewert, and J. C. Weill. 1990. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 9:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 5.Debrauwere, H., S. Loeillet, W. Lin, J. Lopes, and A. Nicolas. 2001. Links between replication and recombination in Saccharomyces cerevisiae: a hypersensitive requirement for homologous recombination in the absence of Rad27 activity. Proc. Natl. Acad. Sci. USA 98:8263-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Noia, J. M., and M. S. Neuberger. 2004. Immunoglobulin gene conversion in chicken DT40 cells largely proceeds through an abasic site intermediate generated by excision of the uracil produced by AID-mediated deoxycytidine deamination. Eur. J. Immunol. 34:504-508. [DOI] [PubMed] [Google Scholar]
- 7.Elliott, B., and M. Jasin. 2002. Double-strand breaks and translocations in cancer. Cell. Mol. Life Sci. 59:373-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, B., C. Richardson, J. Winderbaum, J. A. Nickoloff, and M. Jasin. 1998. Gene conversion tracts from double-strand break repair in mammalian cells. Mol. Cell. Biol. 18:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedberg, E. C., G. C. Walker, and W. Siede (ed.). 1995. DNA repair and mutagenesis. ASM Press, Washington, D.C.
- 10.Fukagawa, T., Y. Mikami, A. Nishihashi, V. Regnier, T. Haraguchi, Y. Hiraoka, N. Sugata, K. Todokoro, W. Brown, and T. Ikemura. 2001. CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J. 20:4603-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima, T., M. Takata, C. Morrison, R. Araki, A. Fujimori, M. Abe, K. Tatsumi, M. Jasin, P. K. Dhar, E. Sonoda, T. Chiba, and S. Takeda. 2001. Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J. Biol. Chem. 276:44413-44418. [DOI] [PubMed] [Google Scholar]
- 12.Haber, J. E. 1998. The many interfaces of Mre11. Cell 95:583-586. [DOI] [PubMed] [Google Scholar]
- 13.Haber, J. E. 2000. Partners and pathways; repairing a double-strand break. Trends Genet. 16:259-264. [DOI] [PubMed] [Google Scholar]
- 14.Harrington, J. J., and M. R. Lieber. 1994. The characterization of a mammalian DNA structure-specific endonuclease. EMBO J. 13:1235-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochegger, H., E. Sonoda, and S. Takeda. 2004. Post-replication repair in DT40 cells: translesion polymerases versus recombinases. BioEssays 26:151-158. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, R. D., and M. Jasin. 2000. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 19:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura, S., T. Suzuki, Y. Yanagawa, T. Yamamoto, H. Nakagawa, I. Tanaka, J. Hashimoto, and K. Sakaguchi. 2001. Characterization of plant proliferating cell nuclear antigen (PCNA) and flap endonuclease-1 (FEN-1), and their distribution in mitotic and meiotic cell cycles. Plant J. 28:643-653. [DOI] [PubMed] [Google Scholar]
- 18.Kucherlapati, M., K. Yang, M. Kuraguchi, J. Zhao, M. Lia, J. Heyer, M. F. Kane, K. Fan, R. Russell, A. M. Brown, B. Kneitz, W. Edelmann, R. D. Kolodner, M. Lipkin, and R. Kucherlapati. 2002. Haploinsufficiency of flap endonuclease (Fen1) leads to rapid tumor progression. Proc. Natl. Acad. Sci. USA 99:9924-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen, E., C. Gran, B. E. Saether, E. Seeberg, and A. Klungland. 2003. Proliferation failure and gamma radiation sensitivity of Fen1 null mutant mice at the blastocyst stage. Mol. Cell. Biol. 23:5346-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis, L. K., G. Karthikeyan, J. W. Westmoreland, and M. A. Resnick. 2002. Differential suppression of DNA repair deficiencies of yeast rad50, mre11, and xrs2 mutations by EXO1 and TLC1 (the RNA component of telomerase). Genetics 160:49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieber, M. R. 1997. The FEN-1 family of structure-specific nucleases in eukaryotic DNA replication, recombination and repair. BioEssays 19:233-240. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki, Y., N. Adachi, and H. Koyama. 2002. Vertebrate cells lacking FEN-1 endonuclease are viable but hypersensitive to methylating agents and H2O2. Nucleic Acid Res. 30:3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazin, A. V., E. Zaitseva, P. Sung, and S. C. Kowalczykowski. 2000. Tailed duplex DNA is the preferred substrate for Rad51 protein-mediated homologous pairing. EMBO J. 19:1148-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreau, S., E. A. Morgan, and L. S. Symington. 2001. Overlapping functions of the Saccharomyces cerevisiae Mre11, Exo1 and Rad27 nucleases in DNA metabolism. Genetics 159:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negritto, M. C., J. Qiu, D. O. Ratay, B. Shen, and A. M. Bailis. 2001. Novel function of Rad27 (FEN-1) in restricting short-sequence recombination. Mol. Cell. Biol. 21:2349-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paques, F., and J. E. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63:349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish, J. Z., C. Yang, B. Shen, and D. Xue. 2003. CRN-1, a Caenorhabditis elegans FEN-1 homologue, cooperates with CPS-6/EndoG to promote apoptotic DNA degradation. EMBO J. 22:3451-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranalli, T. A., S. Tom, and R. A. Bambara. 2002. AP endonuclease 1 coordinates flap endonuclease 1 and DNA ligase I activity in long patch base excision repair. J. Biol. Chem. 277:41715-41724. [DOI] [PubMed] [Google Scholar]
- 29.Reynaud, C. A., B. Bertocci, A. Dahan, and J. C. Weill. 1994. Formation of the chicken B-cell repertoire: ontogenesis, regulation of Ig gene rearrangement, and diversification by gene conversion. Adv. Immunol. 57:353-378. [DOI] [PubMed] [Google Scholar]
- 30.Sale, J. E., D. M. Calandrini, M. Takata, S. Takeda, and M. S. Neuberger. 2001. Ablation of XRCC2/3 transforms immunoglobulin V gene conversion into somatic hypermutation. Nature 412:921-926. [DOI] [PubMed] [Google Scholar]
- 31.Sonoda, E., C. Morrison, Y. M. Yamashita, M. Takata, and S. Takeda. 2001a. Reverse genetic studies of homologous DNA recombination using the chicken B-lymphocyte line, DT40. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda, E., M. S. Sasaki, C. Morrison, Y. Yamaguchi-Iwai, M. Takata, and S. Takeda. 1999. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol. 19:5166-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonoda, E., M. Takata, Y. M. Yamashita, C. Morrison, and S. Takeda. 2001. Homologous DNA recombination in vertebrate cells. Proc. Natl. Acad. Sci. USA 98:8388-8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takata, M., M. S. Sasaki, E. Sonoda, C. Morrison, M. Hashimoto, H. Utsumi, Y. Yamaguchi-Iwai, A. Shinohara, and S. Takeda. 1998. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 17:5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takata, M., M. S. Sasaki, E. Sonoda, T. Fukushima, C. Morrison, J. S. Albala, S. M. Swagemakers, R. Kanaar, L. H. Thompson, and S. Takeda. 2000. The Rad51 paralog Rad51B promotes homologous recombinational repair. Mol. Cell. Biol. 20:6476-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takata, M., M. S. Sasaki, S. Tachiiri, T. Fukushima, E. Sonoda, D. Schild, L. H. Thompson, and S. Takeda. 2001. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralog. Mol. Cell. Biol. 21:2858-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tauchi, H., J. Kobayashi, K. Morishima, D. C. van Gent, T. Shiraishi, N. S. Verkaik, D. vanHeems, E. Ito, A. Nakamura, E. Sonoda, M. Takata, S. Takeda, S. Matsuura, and K. Komatsu. 2002. Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 420:93-98. [DOI] [PubMed] [Google Scholar]
- 38.Tishkoff, D. X., A. L. Boerger, P. Bertrand, N. Filosi, G. M. Gaida, M. F. Kane, and R. D. Kolodner. 1997a. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exo nuclease that interacts with MSH2. Proc. Natl. Acad. Sci. USA 94:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tishkoff, D. X., N. Filosi, G. M. Gaida, and R. D. Kolodner. 1997b. A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell 88:253-263. [DOI] [PubMed] [Google Scholar]
- 40.Tomita, K., A. Matsuura, T. Caspari, A. M. Carr, Y. Akamatsu, H. Iwasaki, K. Mizuno, K. Ohta, M. Uritani, T. Ushimaru, K. Yoshinaga, and M. Ueno. 2003. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol. Cell. Biol. 23:5186-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsubouchi, H., and H. Ogawa. 2000. Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol. Biol. Cell 11:2221-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turchi, J. J., L. Huang, R. S. Murante, Y. Kim, and R. A. Bambara. 1994. Enzymatic completion of mammalian lagging-strand DNA replication. Proc. Natl. Acad. Sci. USA 91:9803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.S. Waga, G. Bauer, and B. Stillman. 1994. Reconstitution of complete SV40 DNA replication with purified replication factors. J. Biol. Chem. 269:10923-10934. [PubMed] [Google Scholar]
- 44.Wei, K., A. B. Clark, E. Wong, M. F. Kane, D. J. Mazur, T. Parris, N. K. Kolas, R. Russell, H. Hou, Jr., B. Kneitz, G. Yang, T. A. Kunkel, R. D. Kolodner, P. E. Cohen, and W. Edelmann. 2003. Inactivation of exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 17:603-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West, S. C. 2003. Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell. Biol. 4:435-445. [DOI] [PubMed] [Google Scholar]
- 46.White, C. I., and J. E. Haber. 1990. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 9:663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, X., T. E. Wilson, and M. R. Lieber. 1999. A role for FEN-1 in nonhomologous DNA end joining: the order of strand annealing and nucleolytic processing events. Proc. Natl. Acad. Sci. USA 96:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi-Iwai, Y., E. Sonoda, M. S. Sasaki, C. Morrison, T. Haraguchi, Y. Hiraoka, Y. M. Yamashita, T. Yagi, M. Takata, C. Price, N. Kakazu, and S. Takeda. 1999. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 18:6619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita, Y. M., T. Okada, T. Matsusaka, E. Sonoda, G. Y. Zhao, K. Araki, S. Tateishi, M. Yamaizumi, and S. Takeda. 2002. RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. EMBO J. 21:5558-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazoe, M., E. Sonoda, H. Hochegger, and S. Takeda. 2004. Reverse genetic studies of the DNA damage response in the chicken B lymphocyte line DT40. DNA Repair 3:1175-1185. [DOI] [PubMed] [Google Scholar]
- 51.Zheng, L., M. Zhou, Q. Chai, J. Parrish, D. Xue, S. M. Patrick, J. J. Turchi, S. M. Yannone, D. Chen, and B. Shen. 2005. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep. 6:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.