Abstract
Mitogen-activated protein kinase pathways are implicated in the regulation of cell differentiation, although their precise roles in many differentiation programs remain elusive. The Raf/MEK/extracellular signal-regulated kinase (ERK) kinase cascade has been proposed to both promote and inhibit adipogenesis. Here, we titrate expression of the molecular scaffold kinase suppressor of Ras 1 (KSR1) to regulate signaling through the Raf/MEK/ERK/p90 ribosomal S6 kinase (RSK) kinase cascade and show how it determines adipogenic potential. Deletion of KSR1 prevents adipogenesis in vitro, which can be rescued by introduction of low levels of KSR1. Appropriate levels of KSR1 coordinate ERK and RSK activation with C/EBPβ synthesis leading to the phosphorylation and stabilization of C/EBPβ at the precise moment it is required within the adipogenic program. Elevated levels of KSR1 expression, previously shown to enhance cell proliferation, promote high, sustained ERK activation that phosphorylates and inhibits peroxisome proliferator-activated receptor gamma, inhibiting adipogenesis. Titration of KSR1 expression reveals how a molecular scaffold can modulate the intensity and duration of signaling emanating from a single pathway to dictate cell fate.
The Raf/MEK/extracellular signal-regulated kinase (ERK) kinase cascade is an evolutionarily conserved pathway involved in the determination of cell fate (50, 86). In mammalian cells, signaling through the Raf/MEK/ERK kinase cascade has been implicated in multiple aspects of cell fate determination, including the regulation of senescence, proliferation, transformation, differentiation, and apoptosis (50). While a positive role for ERK signaling is well established in proliferation, transformation, and oncogene-induced senescence (29, 47, 79, 90), its role in cell differentiation programs remains controversial. ERK activation has been shown to play both positive and negative roles in T-cell commitment (2, 5, 12, 32, 64), myogenesis (19, 51), and adipogenesis (17, 20, 53, 62, 71), with the results seemingly dependent upon the methodology used to study the Raf/MEK/ERK kinase cascade. In adipogenic conversion of 3T3-L1 preadipocytes, inhibition of pathway activity reveals a positive role for ERKs (53, 62, 71), whereas constitutive activation of the pathway suggests a negative role for ERKs (17, 20).
Preadipocyte differentiation is influenced by endocrine and autocrine factors that promote or constrain adipogenesis by intracellular mechanisms that induce the synthesis and activation of adipogenic transcription factors (58). Upon treatment of growth-arrested fibroblasts with a hormonal cocktail of methylisobutylxanthine, dexamethasone, and insulin (MDI), there is a rapid induction of C/EBPβ and C/EBPδ (1 to 2 h) lasting 2 to 3 days (69, 70, 83). With the expression of C/EBPβ/δ, postconfluent, growth-arrested preadipocytes reenter the cell cycle and undergo multiple rounds of cellular division, a process termed mitotic clonal expansion (MCE) (70, 71). C/EBPβ/δ then induce the expression of C/EBPα and peroxisome proliferator-activated receptor gamma (PPARγ) (56, 82-84). C/EBPα and PPARγ terminate MCE and together induce the expression of genes involved in triglyceride storage and metabolism that lead to formation of a mature adipocyte (56-58, 83, 84).
C/EBPβ is necessary for adipogenic conversion of cultured cells. Fibroblasts from C/EBPβ−/− C/EBPδ−/− or C/EBPβ−/− mice fail to differentiate into adipocytes (69, 70). C/EBPβ is necessary for MCE and induction of C/EBPα and PPARγ (70, 82, 85). Expression of C/EBPβ is controlled transcriptionally by CREB (4, 89). Work in multiple cell systems suggests, however, that C/EBPβ activity is controlled posttranslationally by multiple kinases (8, 27, 42, 52). Phosphorylation of C/EBPβ by the Raf/MEK/ERK/p90 ribosomal S6 kinase (RSK) kinase cascade regulates its stability and activity. Phosphorylation of Thr217 by RSK inactivates a caspase-inhibitory box on C/EBPβ, increasing its stability and thereby enhancing its expression and activity (8, 35). Phosphorylation of Thr188 by ERK transactivates C/EBPβ (27, 42, 52).
While the Raf/MEK/ERK kinase cascade is thought to play an important role in adipogenic conversion, its precise contribution remains controversial. Studies using the specific MEK inhibitor U0126 or antisense DNA against ERK indicate that the activation of ERK and CREB is necessary for the induction of C/EBPβ/δ, for MCE, and for the induction of C/EBPα and PPARγ (4, 53, 62, 71). Conversely, activation of ERK with constitutively active upstream effectors causes ERK-mediated phosphorylation and inactivation of PPARγ and blocks terminal differentiation (1, 10, 17, 20, 88). These observations have led to disparate conclusions that ERKs function to promote and inhibit adipocyte differentiation.
Kinase suppressor of Ras 1 (KSR1) (25, 68, 73) is a scaffold for the Raf/MEK/ERK kinase cascade that regulates the activation of Raf by Ras (36, 74) and the activation of MEK by Raf (34, 39). Consistent with the predicted effects of a scaffold on its cognate signaling cassette (9, 28), KSR1 interacts with Raf, MEK, and ERK (22, 26, 36, 61, 66), and its deletion impairs the activation of ERK by growth factors and serum (26). Experimental manipulation of KSR1 expression regulates the degree of ERK activation with coincident effects on the proliferative rate and oncogenic potential of cells (26). The ability to manipulate KSR1 expression and, in turn, control signal transduction through the Raf/MEK/ERK kinase cascade provided the opportunity to examine the role of ERK activation in regulating cell fate in greater detail.
Two important questions arise when the role of the Raf/MEK/ERK cascade in cell fate determination is considered: (i) how can activation of this pathway both activate and inhibit the same cellular program and (ii) how can a single kinase cascade give rise to multiple cell fates? We examine these questions by titrating expression of the molecular scaffold KSR1 and examining its effect on signal transduction through the Raf/MEK/ERK kinase cascade to regulate adipogenesis. We show that moderate levels of KSR1 are required to coordinate Raf/MEK/ERK/RSK signaling with C/EBPβ expression and ensure progression of the adipogenic program. Titration of KSR1 to higher levels prolongs ERK activation and enhances mitogenesis (26) but inhibits adipogenesis by promoting ERK-mediated inactivation of the antiproliferative transcription factor PPARγ. Thus, the intensity and duration of Raf/MEK/ERK signaling determine the biologic consequence of pathway activity to affect adipogenic potential. Manipulating KSR1 directs pathway activity and cell fate.
MATERIALS AND METHODS
Mice.
DBA1/LacJ KSR1−/− mice were previously described (43). Male KSR1−/− and KSR1+/+ mice were fed a chow diet (4% fat) ad libitum, and weights were recorded every 4 weeks until 20 weeks of age. Mice were then sacrificed, and epididymal, inguinal, visceral, and brown adipose fat pads were dissected and weighed (n = 6 mice in each group).
Analysis of adipocyte size.
Sections were obtained from epididymal fat pads of 20-week-old male KSR1−/− and KSR1+/+ mice (n = 6 mice in each group). The fat pads were fixed and stained with hematoxylin and eosin. For each mouse, ×20 photomicrographs from three independent fields containing 50 to 100 adipocytes were analyzed using IPLab software (Scanalytics Inc., Fairfax, VA).
Plasmids.
KSR1 plasmids were described previously (26). MSCV-IRES-YFP was created by substitution of yellow fluorescent protein (YFP) for green fluorescent protein (GFP) in MSCV-IRES-GFP (a gift from Eric Gosner, St. Jude's Children's Hospital, Memphis, TN). Wild-type PPARγ (a gift from Bruce Spiegelman, Harvard Medical School, Boston, MA) was subcloned into MSCV-IRES-YFP. PPARγS112A-IRES-YFP was produced by site-directed mutagenesis of PPARγ-IRES-YFP. pLXSN C/EBPα was a gift from Ormond MacDougald (University of Michigan, Ann Arbor, MI). C/EBPβ was subcloned into the pWZL retroviral vector containing blasticidin S resistance (a gift from Gary Nolan, Stanford University, Palo Alto, CA).
Cell lines.
Primary mouse embryo fibroblasts (MEFs) were derived from KSR1+/+ and KSR1−/− embryos at embryonic day 13.5, as described previously (43). Immortalized MEFs were derived by passaging primary KSR1−/− and KSR1+/+ MEFs under a 3T9 protocol (75). KSR1−/− and KSR1+/+ MEFs and KSR1−/− MEFs expressing ectopic KSR1 were described previously (26, 43). KSR1−/− and KSR1+/+ MEFs expressing C/EBPβ were produced by retroviral infection and selection using 10 μg/ml blasticidin S for 7 days. KSR1−/− cells expressing both KSR1 and PPARγ were produced by infection with bicistronic retroviruses encoding KSR1-IRES-GFP and PPARγ-IRES-YPF or control viruses and sorted for green and yellow fluorescence by fluorescence-activated cell sorter analysis. Cells were excited at 488 nm, and GFP and YFP signals were discriminated with a 525-nm short-pass dichroic filter. Cells demonstrating appropriate levels of fluorescence through both 530/30-nm and 550/30-nm filters were collected.
Cell culture and retroviral infection.
Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 0.1 mM minimal essential medium nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated at 37°C in 5% CO2. To produce recombinant retroviruses, retroviral vectors were cotransfected with an ecotropic packaging vector (a gift from Tom Smithgall, University of Pittsburgh School of Medicine, Pittsburgh, PA) into 293T cells, and supernatants were collected 48 to 72 h posttransfection. Retroviral infection of MEFs was performed using 8 μg/ml polybrene.
Preparation of cell extracts and Western blotting.
Cells were lysed in Triton-sodium dodecyl sulfate (SDS) lysis buffer (40 mM Tris [pH 7.4], 120 mM NaCl, 0.5% Triton X-100, 0.3% SDS, 10 mM sodium pyrophosphate, 2 mM EGTA, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, 5 mg/ml aprotinin). Twenty-five micrograms of total protein was loaded per well, and lysates were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting. Antibodies were from Santa Cruz unless otherwise noted: anti-PPARγ (Upstate), anti-RSK (Transduction Laboratories), anti-phospho-RSK (Ser380) (Cell Signaling), anti-phospho-ERK1/2 antibodies (Cell Signaling), anti-KSR1 (Transduction Laboratories), anti-Foxo1 (Cell Signaling), anti-phospho-Foxo1 (Ser256) (Cell Signaling), anti-STAT5 (Cell Signaling), and anti-actin (Sigma). Anti-mouse, anti-rabbit, and anti-goat secondary antibodies conjugated to Alexa Fluor 680 (1:3,000; Molecular Probes) and IRDye800 (1:3,000; Rockland) were used to probe primary antibodies. Protein bands were detected and quantified on a Li-Cor Odyssey infrared imaging system. To assess PPARγ phosphorylation, samples were run on an 11% acrylamide-bis (100:1) gel containing 5 M urea (20).
Adipogenesis.
Primary or immortalized MEFs were seeded in 35-mm dishes and grown to 2 days postconfluence (day 0). Cells were then stimulated with insulin (4 μg/ml; Sigma), dexamethasone (10 ng/ml; Sigma), and methylisobutylxanthine (115 μg/ml; Sigma) in 10% fetal bovine serum (FBS) for 2 days and insulin in 10% FBS for an additional 2 days before being transferred into 10% FBS for the remainder of the treatment (67). For primary MEFs, cells were also stimulated with pioglitazone (5 μM; Sigma) for 4 days. MCE was assessed at day 0 and day 3 by trypan blue cell counting. Samples were lysed at various times throughout MDI stimulation for Western blot analysis. For C/EBPβ stability, 2-day postconfluent cells were treated with MDI for 4 hours in 10% FBS and then shifted to 10% FBS containing 50 μg/ml cycloheximide for the indicated times. To inhibit ERK activity in KSR1-expressing MEFs, U0126 (Cell Signaling) was added at various concentrations to the differentiation medium 4 h following MDI stimulation and was maintained in the culture medium for 8 days.
In situ ERK activation assay.
Cells were seeded at 1.5 × 104 cells/well in a 96-well plate 24 h prior to analysis and subjected to an in situ plate assay using the Li-Cor Odyssey infrared imaging system to quantify ERK activation. Cells at 70% confluence were deprived of serum for 4 hours and treated with 25 ng/ml platelet-derived growth factor (PDGF) in Dulbecco's modified Eagle's medium plus 1% bovine serum albumin for 5 min. Anti-phospho-ERK1/2 (1:100; Cell Signaling) and anti-ERK1 (1:100; Santa Cruz) primary antibodies and anti-mouse Alexa Fluor 680 (1:100; Molecular Probes) and anti-rabbit IRDye800 (1:100; Rockland) secondary antibodies were used to detect and quantify phosphorylated and total ERK protein levels.
RT-PCR.
Total RNA was prepared from cells or tissues with TRI reagent (Molecular Research Center) according to the manufacturer's instructions. Five micrograms of total RNA was reverse transcribed with Superscript II reverse transcriptase (Invitrogen), and 1/20 of the reverse transcription (RT) reaction was used for PCR. The sequences of primers were as follows: KSR-1 forward, 5′-GGCAAAGCTGGTGAAATAC-3′; KSR-1 reverse, 5′-AGCTACTGTCTCGAGCATCT-3′; KSR-2 forward, 5′-CTGTCCTGCAAGAAGAAGGTG-3′; KSR-2 reverse, 5′-GCACGTCTGAGACATCCAGTA-3′; GAPDH forward, 5′-CCCTTCATTGACCTCAACTAC-3′; GAPDH reverse, 5′-TACTCCTTGGAGGCCATGT-3′.
Northern blot analysis.
RNA was isolated with TRI reagent (Molecular Research Center) according to the manufacturer's protocol. Total RNA (20 μg) was separated in 1% agarose-formaldehyde gels, transferred to Zeta-Probe GT membrane (Bio-Rad), and probed with [32P]dCTP-radiolabeled DNA. A full-length C/EBPβ vector, a 600-bp mouse KSR1, and a 700-bp β-2-microglobulin cDNA fragment were randomly primed with the DECAprime II kit (Ambion) according to the manufacturer's specifications. The blots were hybridized with ULTRAhyb solution (Ambion) at 50°C overnight. Following hybridization, Northern blots were washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS at room temperature for 10 min and twice again with 0.1× SSC-0.1% SDS at 50°C for 15 min. Hybridized Northern blots were analyzed by storage phosphor technology (Molecular Dynamics).
Statistical analysis.
All values are means ± standard deviations (SD) unless otherwise stated. Statistical analyses were carried out using a two-tailed Student's t test. Significance was rejected at a P value of ≥0.05.
RESULTS
KSR1−/− mice have enlarged adipocytes.
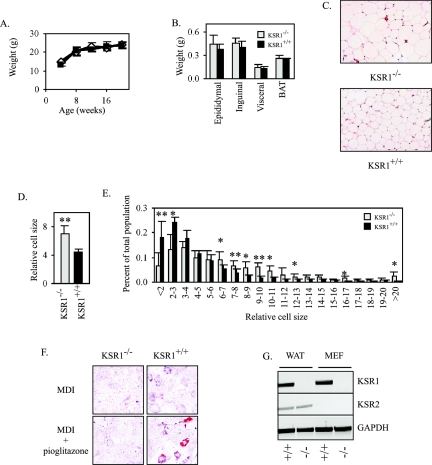
KSR1−/− mice are overtly normal and fertile and grow at the same rate as their wild-type littermates (Fig. 1A), although cells from these mice show diminished ERK activation (30, 43). The masses of fat depots in KSR1−/− mice and their wild-type littermates were also indistinguishable (Fig. 1B). However, histological examination of epididymal adipose tissue revealed a marked increase in the cross-sectional area of adipocytes in KSR1−/− mice compared to KSR1+/+ mice. (Fig. 1C and D). Analysis of size distribution of epididymal adipocytes demonstrated that while KSR1+/+ mice had significantly more of the smallest detectable cells, KSR1−/− mice had more cells that were 2 to 10 times larger in the cross-sectional area (Fig. 1E). Considering the identical fat pad weights for KSR1−/− and KSR1+/+ mice, these data imply that KSR1−/− mice have fewer, but larger, adipocytes.
FIG. 1.
KSR1−/− mice have enlarged adipocytes. (A) Weights of KSR1−/− (open diamonds) and KSR1+/+ (closed squares) mice fed a standard chow diet ad libitum. Values are the means ± SD (n = 6 mice). (B) Weights of different fat depots from 20-week-old KSR1−/− (gray) and KSR1+/+ (black) mice as described above (A). Values are the means ± SD (n = 6 mice). (C) Representative photomicrographs (×20) of epididymal fat pads as described above (B). (D) Average cross-sectional area of epididymal adipocytes from fat pads dissected as described above (B). Three independent fields from six KSR1−/− and six KSR1+/+ mice were analyzed as described in Materials and Methods. Values are the means ± SD. ** denotes a P value of ≤0.01. (E) Distribution curve of adipocyte size analyzed as described above (D). Values are the means ± SD. * denotes a P value of ≤0.05; ** denotes a P value of ≤0.01. (F) Oil Red O staining of primary KSR1−/− and KSR1+/+ MEFs 8 days following treatment with differentiation medium to induce adipogenesis as described in Materials and Methods. (G) Ethidium bromide-stained gel of RT-PCR products showing expression of KSR1, KSR2, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcripts in white adipose tissue (WAT) or MEFs from KSR1−/− and KSR1+/+ mice.
The reduced number of adipocytes in fat depots of KSR1−/− mice could be due to a reduction in the adipogenic capacity of KSR1−/− preadipocytes to differentiate. To test their adipogenic potential, primary MEFs from KSR1−/− and KSR1+/+ mice were treated with an adipogenic cocktail of MDI with or without the synthetic PPARγ ligand pioglitazone. Scattered adipocytes were detected by Oil Red O staining in only KSR1+/+ MEFs treated with the adipogenic cocktail and pioglitazone (Fig. 1F). The absence of detectable differentiation in KSR1−/− MEFs suggested a role for KSR1 in adipogenesis of MEFs. However, this defect was relatively severe in comparison to the decrease in adipocyte number and increase in adipocyte size caused by KSR1 deletion in vivo. A related gene, KSR2, has been recently identified in Caenorhabditis elegans (45) and humans (11). We examined white adipose tissue and MEFs from KSR1−/− and KSR1+/+ mice for the expression of KSR1 and KSR2. KSR2 was detected in both adipocytes from KSR1−/− and KSR1+/+ mice but was undetected in KSR1−/− or KSR1+/+ MEFs (Fig. 1G). Thus, KSR2 expression may minimize the effect of KSR1 deletion in vivo, while its absence in KSR1−/− MEFs may deprive cells in culture of a family of effectors necessary for hormonally induced adipogenesis.
KSR1 is necessary for adipogenic progression.
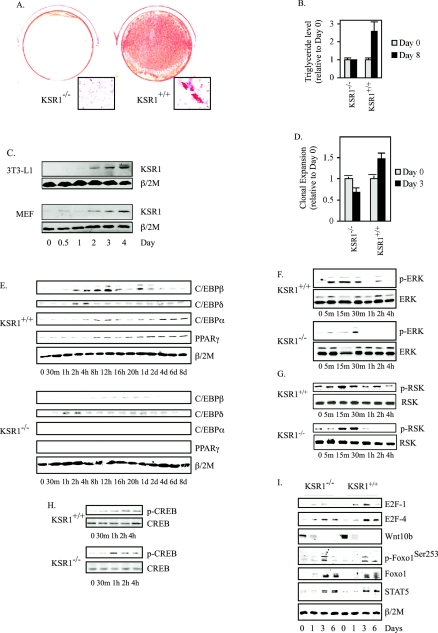
To determine the contribution of KSR1-scaffolded signaling to adipogenesis, postconfluent, immortalized KSR1−/− and KSR1+/+ MEFs were treated with MDI to induce differentiation. KSR1 was necessary for adipogenic conversion of fibroblasts, as MDI-treated KSR1+/+ but not KSR1−/− MEFs showed substantial triglyceride accumulation following 8 days of treatment (Fig. 2A and B). An increase in KSR1 expression coincides with the differentiation of HL-60 cells (78) and PC12 cells (66). We examined whether KSR1 expression was altered similarly during adipogenesis. When either 3T3-L1 preadipocytes or wild-type mouse embryo fibroblasts were treated with MDI, the level of KSR1 protein increased throughout the first 4 days of adipogenic induction (Fig. 2C). Ectopic expression of KSR1 in KSR1−/− MEFs elevates ERK activity and enhances cell proliferation in a dose-dependent manner (26).
FIG. 2.
KSR1 is necessary for C/EBPβ induction and adipogenesis. (A) Oil Red O staining of immortalized KSR1−/− and KSR1+/+ MEFs 8 days following treatment with differentiation medium to induce adipogenesis as described in Materials and Methods. (B) Analysis of triglyceridelevels in immortalized KSR1−/− and KSR1+/+ MEFs 0 and 8 days after treatment with differentiation medium. Values are the means ± SD of four independent experiments. (C) Western blot analysis of whole-cell extracts prepared at different times during differentiation of 3T3-L1 preadipocytes and immortalized KSR1+/+ MEFs. Lysates were probed with an anti-KSR1 antibody to detect induction of KSR1 during adipogenesis. β/2 microglobulin (β/2 M) was used to demonstrate equal loading of each sample. (D) Analysis of MCE in KSR1−/− and KSR1+/+ MEFs by counting cell number 0 and 3 days after treatment with differentiation medium. Values are the means ± SD of four independent experiments. (E to I) Western blot analysis of whole-cell extracts prepared at different times during differentiation of KSR1−/− and KSR1+/+ MEFs. Lysates were probed with the indicated antibodies to detect induction and activation of each protein during adipogenesis. β/2 microglobulin (β/2 M) was used to demonstrate equal loading of each sample.
We tested whether deletion of KSR1 would affect the MCE of preadipocytes that accompanies adipocyte differentiation in vitro. Though controversial (15, 54), MCE of preadipocytes has been suggested as a precursor to acquisition of the terminal adipogenic program (31, 49). MCE was assessed in wild-type and KSR1−/− MEFs 3 days after MDI treatment, and Western blotting was performed for C/EBPβ, C/EBPδ, C/EBPα, and PPARγ from 0 to 8 days. KSR1 was necessary for induction of MCE (Fig. 2D), consistent with data implicating MEK activation in this process (71). Western blot analysis revealed that deletion of KSR1 inhibited maximal induction of C/EBPβ and completely eliminated induction of its downstream effectors C/EBPα and PPARγ. Induction of C/EBPδ was unaffected by KSR1 deletion (Fig. 2E). C/EBPβ expression is dependent upon the coordinated action of the Raf/MEK/ERK/RSK and CREB pathways (4, 89). CREB phosphorylation was unaffected in MDI-treated KSR1−/− MEFs (Fig. 2H), indicating that CREB does not play a critical role in regulating the effect of KSR1 on adipogenesis. Although ERK and RSK were both phosphorylated in MDI-treated KSR1−/− MEFs, sustained activation of each kinase was abrogated (Fig. 2F and G). Adipogenesis is also regulated by the action of a number of additional effectors, including E2F-1, Wnt10b, Foxo1, and STAT5 (16, 41, 59, 65). However, expression and/or phosphorylation of these effectors in the adipogenic program is unaltered by the deletion of KSR1 (Fig. 2I), suggesting that these proteins and the pathways they regulate function independently of KSR1 and the Raf/MEK/ERK kinase cascade.
Ectopic C/EBPβ expression restores adipogenic potential to KSR1−/− MEFs.
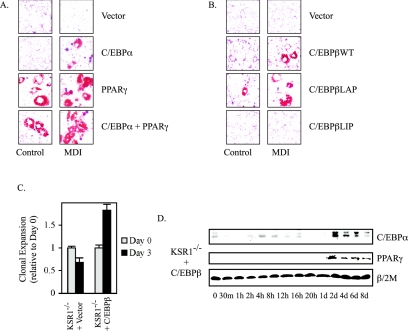
Deletion of C/EBPβ, C/EBPα, or PPARγ blocks adipogenic conversion of fibroblasts (56, 57, 69, 70, 84). To determine whether the impaired adipogenesis of KSR1−/− MEFs was due to the loss of transcription factor expression, C/EBPβ, C/EBPα, or PPARγ was introduced to KSR1−/− MEFs. Introduction of C/EBPα or PPARγ rescued adipogenesis in KSR1−/− MEFs as assessed by Oil Red O staining (Fig. 3A). PPARγ permitted MDI-independent differentiation and C/EBPα allowed MDI-dependent differentiation, consistent with PPARγ expression being downstream of C/EBPα during adipogenesis (56, 84). These data demonstrate that KSR1−/− MEFs retain the molecular machinery needed to produce mature adipocytes. Furthermore, introduction of C/EBPβ into KSR1−/− MEFs rescued adipogenesis. C/EBPβ expression in KSR1−/− MEFs rescued MDI-induced triglyceride accumulation (Fig. 3B), MCE (Fig. 3C), and expression of C/EBPα and PPARγ (Fig. 3D) compared to control vector expression (Fig. 3B to D and data not shown). C/EBPβ exists as alternate translation products in both an activating form known as liver activating protein (LAP) and an inhibitory form known as liver inhibitory protein (LIP), which lacks the N-terminal transcriptional activation domain found in LAP (13, 14). Constitutive expression of LAP, but not LIP, also promoted adipogenesis in KSR1−/− MEFs and showed the ability to promote adipogenesis in the absence of the hormonal inducers (Fig. 3B), suggesting that KSR1 contributes to adipogenesis by enhancing the function of C/EBPβ.
FIG. 3.
Expression of C/EBPβ, C/EBPα, or PPARγ restores adipogenic conversion in KSR1−/− MEFs. (A and B) Oil Red O staining of KSR1−/− MEFs expressing (A) C/EBPα and/or PPARγ or (B) C/EBPβ (wild type, LAP, or LIP) untreated or following 8 days of treatment with differentiation medium to induce adipogenesis as described in Materials and Methods. (C) Analysis of MCE in KSR1−/− MEFs expressing C/EBPβ or vector control by counting cell numbers 0 and 3 days after treatment with differentiation medium. Values are the means of four independent experiments. (D) Western blot analysis of whole-cell extracts prepared at different times during differentiation of KSR1−/− MEFs expressing C/EBPβ. Lysates were probed with the indicated antibodies to detect induction of each protein during adipogenesis.
KSR1 coordinates RSK activation with C/EBPβ synthesis.
C/EBPβ transcription is controlled by CREB, whereas C/EBPβ stability and activation are modulated by the Raf/MEK/ERK/RSK kinase cascade (4, 8, 27, 42, 48, 52, 89). Northern blot analysis revealed that MDI-stimulated C/EBPβ transcription was similar in both KSR1−/− and KSR1+/+ MEFs (Fig. 4A). These data are consistent with the normal phosphorylation of CREB observed in KSR1−/− MEFs (Fig. 2H).
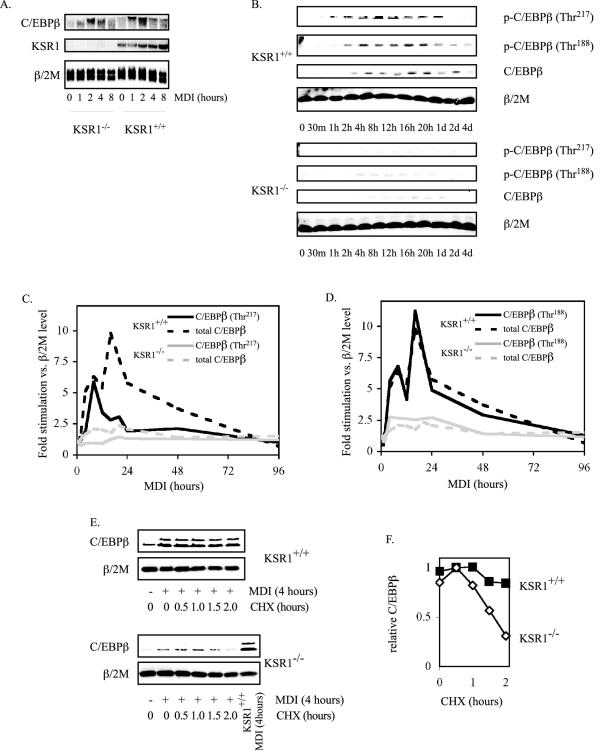
FIG. 4.
KSR1 is required for C/EBPβ phosphorylation and stabilization during adipogenesis. (A) MDI-induced C/EBPβ transcription is independent of KSR1 expression. Shown is Northern blot analysis of C/EBPβ, KSR1, and β/2 M at different times after induction of differentiation in KSR1−/− and KSR1+/+ MEFs. (B) KSR1 enhances C/EBPβ phosphorylation during adipogenesis. Shown is Western blot analysis of whole-cell extracts prepared at different times during differentiation of KSR1−/− and KSR1+/+ MEFs. Lysates were probed with the indicated antibodies to detect induction and activation of C/EBPβ during adipogenesis. (C and D) Total and phosphorylated C/EBPβ expression was quantified from the Western blots described above (B) at each time point and normalized to β/2 M levels, and the resulting data were plotted as fold stimulation following induction of differentiation. Data for Thr217 phosphorylation are shown in C, and data for Thr188 phosphorylation are shown in D. KSR1+/+, black line; KSR1−/−, gray line; phospho-C/EBPβ, solid line; total C/EBPβ, dashed line. Data shown in each panel are the averages of three independent experiments. (E and F) Effect of KSR1 on C/EBPβ stability. Shown is Western blot analysis of whole-cell extracts prepared from KSR1−/− and KSR1+/+ MEFs after 4 h of induction with differentiation medium followed by 0 to 2 h of treatment with cycloheximide (CHX). Lysates were probed for C/EBPβ and β/2 M. C/EBPβ expression at each time point was quantified and normalized to β/2 M levels, and the resulting data were plotted in F. Maximal expression was set arbitrarily to 1. Values are the means of two independent experiments.
Studies in cultured adipocytes, hepatocytes, and neurons have shown that phosphorylation of C/EBPβ on Thr217 by RSK and Thr188 by ERK enhances its stability and transcriptional activity, respectively (8, 27, 35, 42, 48, 52). Upon MDI treatment, newly translated C/EBPβ is phosphorylated on both Thr217 and Thr188 in KSR1+/+ MEFs (Fig. 4B and data not shown), thereby enhancing the stability and transcriptional activity of C/EBPβ. Western blot analyses reveal that both expression and phosphorylation of C/EBPβ are markedly diminished in KSR1−/− MEFs (Fig. 4B and data not shown). Quantitative analysis of total and phosphorylated forms of C/EBPβ reveals that KSR1−/− MEFs are defective in both RSK- and ERK-mediated phosphorylation of C/EBPβ (Fig. 4C and D). Phosphorylation of C/EBPβ at Thr217 peaked within 8 h of MDI treatment in KSR1+/+ MEFs, leading to an increase in C/EBPβ expression (Fig. 4C). Although there was a rapid decline in C/EBPβ phosphorylation after 8 hours, total C/EBPβ levels increased further, most likely due to the ability of C/EBPβ to stimulate its own expression via binding to its promoter in an autoregulatory positive feedback loop (44). RSK-mediated phosphorylation of C/EBPβ on Thr217 was almost absent in KSR1−/− MEFs (Fig. 4B and C and data not shown), which could account for the reduced expression of C/EBPβ (Fig. 2E and 4B and C and data not shown) and the impaired adipogenic potential (Fig. 2A and B) of KSR1−/− MEFs. Phosphorylation of C/EBPβ on Thr188 was also diminished in KSR1−/− MEFs (Fig. 4B and D and data not shown). The reduced phosphorylation and transcriptional activity of C/EBPβ likely contributed to the absence of adipogenesis seen in KSR1−/− MEFs (Fig. 2A and B) despite the low level of delayed C/EBPβ expression (Fig. 2E).
Phosphorylation of C/EBPβ by RSK on Thr217 increases C/EBPβ stability by creating a functional caspase-inhibitory box (8). To examine whether C/EBPβ stability was decreased by KSR1 deletion, KSR1−/− and KSR1+/+ MEFs were treated with MDI for 4 hours and then shifted to medium containing 50 μg/ml cycloheximide for 0 to 2 h. In comparison to KSR1+/+ MEFs, C/EBPβ protein stability was markedly diminished in KSR1−/− MEFs during adipogenic conversion. Two hours after cycloheximide treatment, C/EBPβ expression declined by 15% in MDI-stimulated KSR1+/+ MEFs but by 70% in KSR1−/− MEFs (Fig. 4E and F). These data indicate that KSR1−/− MEFs cannot differentiate into adipocytes due to a lack of sustained RSK activation, the consequence being defective RSK-mediated phosphorylation and stabilization of C/EBPβ.
Levels of KSR1 that enhance mitogenesis inhibit adipogenesis.
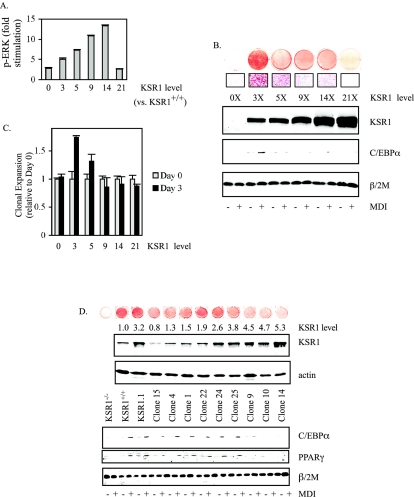
Molecular scaffolds are predicted to affect signaling through their cognate cascades in a concentration-dependent manner (9, 26, 28). Consistent with this concept, growth factor-induced ERK activation and the rate of cell proliferation are dependent upon the amount of KSR1 expressed in cells (26). Induction of adipogenesis in 3T3-L1 preadipocytes and MEFs causes an increase in KSR1 expression (Fig. 2C). To assess the role of KSR1 expression on adipogenic conversion, KSR1−/− MEFs expressing increasing levels of KSR1 (26) were assessed for ERK activation and adipogenic conversion. Introduction of KSR1 into KSR1−/− MEFs rescued adipogenesis in a dose-dependent manner disproportionate to its ability to enhance ERK activation and cell proliferation (26). KSR1 promotes a dose-dependent increase in ERK activation, with maximal ERK activation occurring at 14 times the level of KSR1 expressed in KSR1+/+ MEFs (Fig. 5A). If KSR1 is expressed at higher levels, ERK activation is inhibited via titration of members of the Raf/MEK/ERK kinase cascade (26).
FIG. 5.
Physiologic levels of KSR1 promote adipogenesis. (A) KSR1−/− MEFs expressing increasing levels of KSR1 were serum starved for 4 hours and stimulated with PDGF (25 ng/ml) for 5 min. ERK phosphorylation was assessed by in situ Western blotting as described in Materials and Methods. Data are the means of four independent experiments. (B) Oil Red O staining and Western blot analysis of KSR1−/− MEFs expressing increasing levels of KSR1 8 days following treatment with differentiation medium to induce adipogenesis as described in Materials and Methods. Whole-cell lysates were probed at 0 and 8 days following induction with differentiation medium with the indicated antibodies. KSR1 level compared to KSR1+/+ MEFs is indicated. (C) Analysis of MCE in KSR1−/− MEFs expressing increasing levels of KSR1 by counting cell numbers 0 and 3 days after treatment with differentiation medium. Values are the means of four independent experiments. (D) Oil Red O staining and Western blot analysis of clonal cell populations isolated from the pool of KSR1−/− MEFs averaging three times the KSR1 levels shown in B. Whole-cell lysates were probed with the indicated antibodies at 0 and 8 days following induction with differentiation medium with the indicated antibodies. KSR1 level compared to KSR1+/+ MEFs is indicated.
Expression of KSR1 at three times the level expressed in KSR1+/+ MEFs maximally restored MDI-induced triglyceride accumulation, MCE, and C/EBPα expression (Fig. 5B and C). Higher levels of KSR1 expression, previously shown to enhance proliferation of KSR1−/− MEFs (26), inhibited adipogenesis (Fig. 5B and C). To determine the level of KSR1 expression optimal for adipogenic conversion, clonal cell lines expressing various levels of ectopic KSR1 were isolated from the 3× pool of KSR1-expressing MEFs by single-cell sorting. Analysis of cell lines expressing ectopic KSR1 at 0.8 to 5.3 times wild-type levels revealed that optimal MDI-induced triglyceride accumulation, C/EBPα and PPARγ expression, and MCE occur at two to three times wild-type KSR1 expression levels (Fig. 5D and data not shown).
Elevated levels of KSR1 facilitate phosphorylation and inhibition of PPARγ.
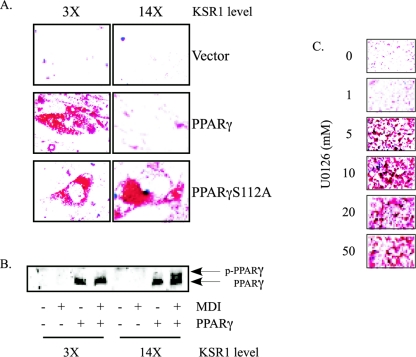
Prolonged ERK activation can cause PPARγ phosphorylation on Ser112, leading to its inactivation and blocking adipogenesis (1, 10, 20, 88). Overexpression of KSR1 promotes sustained ERK activation (26). To determine whether impaired adipogenesis by overexpressing KSR1 was due to the phosphorylation and inactivation of PPARγ, wild-type PPARγ and PPARγS112A, which cannot be phosphorylated by ERK, were introduced into KSR1-expressing MEFs. The resulting cells were then evaluated for spontaneous differentiation following several days of postconfluent culture. Low levels of KSR1 permitted differentiation in MEFs expressing PPARγ or PPARγS112A. However, when KSR was expressed at 14 times the level found in KSR1+/+ MEFs, adipogenesis occurred only in cells expressing PPARγS112A (Fig. 6A). Phosphorylation on Ser112 retards the electrophoretic mobility of PPARγ (20). An MDI-induced shift in PPARγ mobility was observed in KSR1−/− MEFs expressing 14 times, but not 3 times, the levels of KSR1 (Fig. 6B). These data demonstrate that KSR1 expression levels that maximize ERK activation inhibit adipogenesis by inactivating PPARγ via its phosphorylation on Ser112. Consistent with that interpretation, the MEK inhibitor U0126 reverses the inhibitory effects of elevated KSR1 expression in a dose-dependent manner (Fig. 6C). These data implicate ERK activation as the inhibitory signal preventing adipogenesis in cells with high levels of KSR1 expression.
FIG. 6.
Elevated KSR1 expression promotes PPARγ phosphorylation to inhibit adipogenesis. (A) Oil Red O staining of KSR1-expressing MEFs from Fig. 5B infected with retroviruses encoding wild-type PPARγ or PPARγS112A. (B) Western blot analysis of whole-cell extracts of cells from A prepared at days 0 and 8 of differentiation. Lysates were run on a 5 M urea gel (acrylamide-bis; 100:1) and probed with anti-PPARγ antibody to discriminate phosphorylated and nonphosphorylated forms of PPARγ. (C) Oil Red O staining of KSR1-expressing MEFs (14 times the endogenous KSR1 levels) stimulated with MDI and then treated with increasing concentrations of U0126 4 hours following MDI stimulation to inhibit prolonged ERK activation.
DISCUSSION
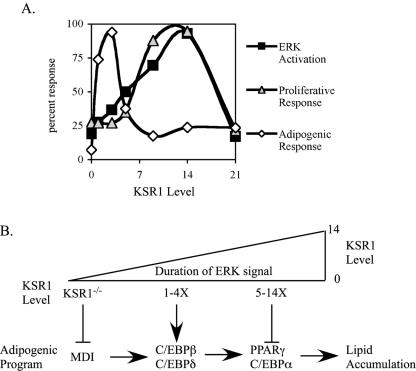
Adipogenesis requires the temporal coordination of multiple signaling cascades. Here, we show that expression of KSR1, a molecular scaffold for the Raf/MEK/ERK kinase cascade, ensures an appropriate level of ERK activity for progression through the adipogenic program. Deletion of KSR1 prevents the phosphorylation and stable expression of the transcription factor C/EBPβ, which is necessary for subsequent expression of C/EBPα and PPARγ and completion of the adipogenic program. However, KSR1 expression levels that promote maximal ERK activation inhibit the adipogenic program by promoting the phosphorylation of PPARγ on a site that inhibits its activity. These data provide an explanation for the conflicting observations of previous reports concluding that ERK activation promotes (4, 53, 62, 71) or inhibits (17, 20) adipogenesis. Adipogenesis requires appropriately timed ERK activation of a measured intensity. Too much, or too little, ERK activation disrupts the efficient activation of transcription factors critical to the adipogenic program (Fig. 7).
FIG. 7.
Distinct KSR1 concentrations promote the proliferative and adipogenic potential of cells. (A) Growth factor-induced ERK activation (closed squares), cell proliferation (gray triangles), and adipogenic response (open diamonds) were plotted as a percent response versus KSR1 expression level. (B) Relationship between KSR1 level, ERK activation, and adipogenic progression. Deletion of KSR1 blocks early steps in adipogenesis by blocking MDI-stimulated ERK activation. Low levels of KSR1 expression (1-4X) promote ERK- and RSK-mediated C/EBPβ phosphorylation, promoting adipogenesis. Higher levels of KSR1 expression (5-14X) inhibit adipogenic progression by ERK-mediated phosphorylation of PPARγ.
These studies demonstrate that KSR1 can coordinate ERK activity with other signaling pathways to regulate the biologic response to ERK activation. We propose that KSR1 provides a mechanism for fine control of ERK signal duration to affect cell fate. KSR1 expression is elevated upon induction of adipogenesis in 3T3-L1 preadipocytes and MEFs (Fig. 2C). Based on previous analyses in KSR1−/− MEFs (26), the induction of endogenous KSR1 in KSR1+/+ MEFs follows a time course that would enhance the duration of ERK signaling during MCE but suppress ERK activation afterward. Recent observations indicate that KSR1 may be regulated by cellular mechanisms affecting its stability or activity. Combined mutation of phosphorylation sites Thr274 and Ser392 in KSR1 enhances its stability and amplifies growth factor-induced ERK activation (55). IMP, a novel E3 ligase, targets KSR1 to a Triton X-100-insoluble fraction to impede the activation of MEK by Raf (34). Although ubiquitination of KSR1 has not been detected, it is reasonable to postulate cellular mechanisms that regulate signaling through the Raf/MEK/ERK signaling cassette by manipulating the stability of a cognate molecular scaffold.
The distribution of KSR1 to different subcellular locations may also regulate signaling through ERK. Phosphorylation by the kinase C-TAK1 and dephosphorylation by protein phosphatase 2A regulate KSR1 translocation to the plasma membrane to facilitate the activation of MEK by Raf (39, 46). KSR1 also translocates to the nucleus in a manner dependent upon its interaction with MEK (7). These observations suggest that KSR1 is dynamically regulated by multiple afferent mechanisms to control signaling through ERK.
The duration of ERK activity can be influenced by external stimuli to regulate differentiation and proliferation (3, 33). Epidermal growth factor (EGF) treatment of PC12 cells causes transient ERK activation and stimulates proliferation, whereas nerve growth factor treatment causes sustained ERK activation, the nuclear translocation of ERK, and neurite outgrowth (21, 76). Ectopic expression of B-KSR1 can modulate the response of PC12 cells to growth factor treatment, promoting higher levels of ERK activity and enhancing neurite development (38). Furthermore, the expression of ectopic B-KSR1 in PC12 cells converts the cellular response to EGF from proliferation to differentiation (38). In fibroblasts, transient ERK activation by EGF is insufficient to stimulate cell proliferation, whereas sustained ERK activation by PDGF promotes proliferation (24, 40). KSR1 expression is necessary for PDGF to promote sustained ERK activation and induce DNA synthesis (26). Furthermore, increasing the level of KSR1 expression converts the cell's response to EGF into a PDGF-like response that promotes proliferation (26). In preadipocytes, endogenous levels of KSR1 facilitate ERK activation at levels sufficient for adipogenic progression. Higher levels of KSR1 expression cause prolonged ERK activation, promoting proliferation at the expense of adipogenesis.
KSR1 may play a similar role in ERK-mediated effects on other differentiation pathways. ERK-mediated phosphorylation of C/EBPα on Ser21 actively inhibits granulopoesis, suggesting that phosphorylation of C/EBPα may be important for directing monocyte versus granulocyte differentiation (60). The effects of allosteric MEK inhibitors or constitutively activated pathway components suggest a potential role for KSR or other scaffolds in regulating ERK signaling in myogenesis (19, 51) and thymocyte maturation (2, 5, 12, 32, 64). Treatment of myoblasts with the MEK inhibitor PD98059 blocks MyoD expression and early proliferative responses necessary for myogenesis (19). In contrast, constitutively active, nuclear MEK can bind directly to MyoD and inhibit its myogenic action (51). In immature thymocytes, high levels of ERK activity are required for clonal deletion of CD4+ CD8+ thymocytes (negative selection), whereas low levels of ERK activity are necessary for maturation of CD4+ CD8+ thymocytes into functional CD4+ or CD8+ T cells (32). Analysis and manipulation of KSR1 or other scaffolds in these cells may reveal novel information about the way in which the Raf/MEK/ERK kinase cascade integrates into other complex differentiation programs.
KSR1−/− mice show a mild phenotype in comparison to the embryonic lethality seen in MEK1 and ERK2 null backgrounds (18, 43, 87). These differences may result from expression of KSR2 (Fig. 1G), a recently identified KSR family member (11, 45). In C. elegans, KSR1 and KSR2 have both overlapping and nonoverlapping functions in response to let60 Ras activation (45). Deletion of KSR1 in MEFs prevents MCE and adipogenesis (Fig. 2) while maintaining proliferative capacity equal to that of KSR1+/+ MEFs (26). The adipose depots of KSR1−/− mice are identical in mass to those of KSR1+/+ mice. This likely reflects the compensatory effects of KSR2 in white adipose tissue (Fig. 1G). Despite the expression of KSR2, deletion of KSR1 limits the number of cells that comprise each depot and causes an increase in average adipocyte size (Fig. 1C to E). This observation may be in vivo evidence of impaired MCE. Consistent with a role for KSR1 in adipogenesis in vivo, ERK1−/− mice have decreased adiposity and fewer adipocytes than wild-type mice (6). Combined deletion of KSR1 and KSR2 should amplify the effects of the scaffold on adipogenesis in vivo. However, deletion of KSR1 and KSR2 in C. elegans is lethal (45) and would be predicted to be so in mice.
Molecular scaffolds are thought to organize kinase cascades to ensure signaling specificity (37). If they reside in specific subcellular compartments, scaffolds may direct a subset of signals emanating from a given signaling cassette. This scaffold-mediated signaling specificity would allow a cell fine control of output from a kinase cascade and provide a point for therapeutic control of one physiological response mediated by an effector without disruption of other responses. Analysis of other scaffolds for mitogen-activated protein (MAP) kinase pathways supports this idea. MP1 and its binding partner, p14, specifically organize MEK1 and ERK1 to coordinate signaling through early endosomes (63, 72). JIP-1, a scaffold for the Jun N-terminal kinase (JNK) pathway, interacts specifically with a subset of MAP kinase kinases and MAP kinase kinase kinases known to activate JNK (80). This specificity of interaction, coupled with the limited tissue distribution of JIP-1 in mice, demonstrates that JIP-1 is not globally required for signals traversing the JNK signaling pathway. Rather, JIP-1 functions specifically in the brain and adipose tissue to mediate JNK responses to ischemic injury (81) and diet-induced obesity (23). OSM, a recently identified scaffold for p38-mediated responses to high osmolarity, provides similar specificity to the p38 pathway for specific stimuli. OSM is necessary for p38 activation by sorbitol but not by other p38 activators such as anisomycin. OSM forms a signaling module with Rac, MEKK3, and MKK3, allowing for stimulus-specific control of p38 activity (77). Although KSR1 binds to both MEK1/2 and ERK1/2, our data suggest that KSR family members regulate a subset of cellular responses to MEK and ERK, specifically those that require precise control of the intensity and duration of their activity, such as proliferation (26), Ras-mediated oncogenesis (26, 30, 43), and adipogenesis (Fig. 1 and 2). Further analysis of KSR family scaffold proteins will define the extent to which they direct cell fate in response to ERK activation.
Acknowledgments
We thank Charles A. Kuszynski and Linda M. Wilkie in the UNMC Cell Analysis Facility for their technical expertise in the generation of GFP- and YFP-expressing cell lines. We thank Michael White and Thomas Smithgall for critical review of the manuscript and helpful advice and encouragement.
This research was supported by NIH grants CA90400 and DK52809 (R.E.L.), the Charlotte Geyer Foundation (R.E.L.), and the American Diabetes Association (R.L.K.).
REFERENCES
- 1.Adams, M., M. J. Reginato, D. Shao, M. A. Lazar, and V. K. Chatterjee. 1997. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 272:5128-5132. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila, J., K. A. Forbush, R. Seger, E. G. Krebs, and R. M. Perlmutter. 1995. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature 373:620-623. [DOI] [PubMed] [Google Scholar]
- 3.Assoian, R. K. 2002. Common sense signalling. Nat. Cell Biol. 4:E187-E18. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte, N., B. W. Phillips, F. Massiera, P. Villageois, B. Wdziekonski, P. Saint-Marc, J. Nichols, J. Aubert, K. Saeki, A. Yuo, S. Narumiya, G. Ailhaud, and C. Dani. 2001. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol. Endocrinol. 15:2037-2049. [DOI] [PubMed] [Google Scholar]
- 5.Bommhardt, U., Y. Scheuring, C. Bickel, R. Zamoyska, and T. Hunig. 2000. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J. Immunol. 164:2326-2337. [DOI] [PubMed] [Google Scholar]
- 6.Bost, F., M. Aouadi, L. Caron, P. Even, N. Belmonte, M. Prot, C. Dani, P. Hofman, G. Pages, J. Pouyssegur, Y. Le Marchand-Brustel, and B. Binetruy. 2005. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 54:402-411. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, J. A., D. J. Volle, O. V. Chaika, and R. E. Lewis. 2002. Phosphorylation regulates the nucleocytoplasmic distribution of kinase suppressor of Ras. J. Biol. Chem. 277:5369-5377. [DOI] [PubMed] [Google Scholar]
- 8.Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. [DOI] [PubMed] [Google Scholar]
- 9.Burack, W. R., and A. S. Shaw. 2000. Signal transduction: hanging on a scaffold. Curr. Opin. Cell Biol. 12:211-216. [DOI] [PubMed] [Google Scholar]
- 10.Camp, H. S., and S. R. Tafuri. 1997. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 272:10811-10816. [DOI] [PubMed] [Google Scholar]
- 11.Channavajhala, P. L., L. Wu, J. W. Cuozzo, J. P. Hall, W. Liu, L. L. Lin, and Y. Zhang. 2003. Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J. Biol. Chem. 278:47089-47097. [DOI] [PubMed] [Google Scholar]
- 12.Crompton, T., K. C. Gilmour, and M. J. Owen. 1996. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86:243-251. [DOI] [PubMed] [Google Scholar]
- 13.Descombes, P., M. Chojkier, S. Lichtsteiner, E. Falvey, and U. Schibler. 1990. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 4:1541-1551. [DOI] [PubMed] [Google Scholar]
- 14.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. [DOI] [PubMed] [Google Scholar]
- 15.Entenmann, G., and H. Hauner. 1996. Relationship between replication and differentiation in cultured human adipocyte precursor cells. Am. J. Physiol. 270:C1011-C1016. [DOI] [PubMed] [Google Scholar]
- 16.Fajas, L., R. L. Landsberg, Y. Huss-Garcia, C. Sardet, J. A. Lees, and J. Auwerx. 2002. E2Fs regulate adipocyte differentiation. Dev. Cell 3:39-49. [DOI] [PubMed] [Google Scholar]
- 17.Font de Mora, J., A. Porras, N. Ahn, and E. Santos. 1997. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3-L1 adipocytic differentiation. Mol. Cell. Biol. 17:6068-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giroux, S., M. Tremblay, D. Bernard, J. F. Cardin-Girard, S. Aubry, L. Larouche, S. Rousseau, J. Huot, J. Landry, L. Jeannotte, and J. Charron. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369-372. [DOI] [PubMed] [Google Scholar]
- 19.Gredinger, E., A. N. Gerber, Y. Tamir, S. J. Tapscott, and E. Bengal. 1998. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 273:10436-10444. [DOI] [PubMed] [Google Scholar]
- 20.Hu, E., J. B. Kim, P. Sarraf, and B. M. Spiegelman. 1996. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274:2100-2103. [DOI] [PubMed] [Google Scholar]
- 21.Huff, K., D. End, and G. Guroff. 1981. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J. Cell Biol. 88:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs, D., D. Glossip, H. Xing, A. J. Muslin, and K. Kornfeld. 1999. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13:163-175. [PMC free article] [PubMed] [Google Scholar]
- 23.Jaeschke, A., M. P. Czech, and R. J. Davis. 2004. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 18:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, S. M., and A. Kazlauskas. 2001. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat. Cell Biol. 3:165-172. [DOI] [PubMed] [Google Scholar]
- 25.Kornfeld, K., D. B. Hom, and H. R. Horvitz. 1995. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell 83:903-913. [DOI] [PubMed] [Google Scholar]
- 26.Kortum, R. L., and R. E. Lewis. 2004. The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol. Cell. Biol. 24:4407-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowenz-Leutz, E., G. Twamley, S. Ansieau, and A. Leutz. 1994. Novel mechanism of C/EBP beta (NF-M) transcriptional control: activation through derepression. Genes Dev. 8:2781-2791. [DOI] [PubMed] [Google Scholar]
- 28.Levchenko, A., J. Bruck, and P. W. Sternberg. 2000. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl. Acad. Sci. USA 97:5818-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozano, J., R. Xing, Z. Cai, H. L. Jensen, C. Trempus, W. Mark, R. Cannon, and R. Kolesnick. 2003. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 63:4232-4238. [PubMed] [Google Scholar]
- 31.Lyle, R. E., V. M. Richon, and R. E. McGehee, Jr. 1998. TNFalpha disrupts mitotic clonal expansion and regulation of retinoblastoma proteins p130 and p107 during 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 247:373-378. [DOI] [PubMed] [Google Scholar]
- 32.Mariathasan, S., A. Zakarian, D. Bouchard, A. M. Michie, J. C. Zuniga-Pflucker, and P. S. Ohashi. 2001. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J. Immunol. 167:4966-4973. [DOI] [PubMed] [Google Scholar]
- 33.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 34.Matheny, S. A., C. Chen, R. L. Kortum, G. L. Razidlo, R. E. Lewis, and M. A. White. 2004. Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature 427:256-260. [DOI] [PubMed] [Google Scholar]
- 35.Menard, C., P. Hein, A. Paquin, A. Savelson, X. M. Yang, D. Lederfein, F. Barnabe-Heider, A. A. Mir, E. Sterneck, A. C. Peterson, P. F. Johnson, C. Vinson, and F. D. Miller. 2002. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron 36:597-610. [DOI] [PubMed] [Google Scholar]
- 36.Michaud, N. R., M. Therrien, A. Cacace, L. C. Edsall, S. Spiegel, G. M. Rubin, and D. K. Morrison. 1997. KSR stimulates Raf-1 activity in a kinase-independent manner. Proc. Natl. Acad. Sci. USA 94:12792-12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91-118. [DOI] [PubMed] [Google Scholar]
- 38.Muller, J., A. M. Cacace, W. E. Lyons, C. B. McGill, and D. K. Morrison. 2000. Identification of B-KSR1, a novel brain-specific isoform of KSR1 that functions in neuronal signaling. Mol. Cell. Biol. 20:5529-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, L. O., S. Smith, R. H. Chen, D. C. Fingar, and J. Blenis. 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4:556-564. [DOI] [PubMed] [Google Scholar]
- 41.Nakae, J., T. Kitamura, Y. Kitamura, W. H. Biggs III, K. C. Arden, and D. Accili. 2003. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4:119-129. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima, T., S. Kinoshita, T. Sasagawa, K. Sasaki, M. Naruto, T. Kishimoto, and S. Akira. 1993. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen, A., W. R. Burack, J. L. Stock, R. Kortum, O. V. Chaika, M. Afkarian, W. J. Muller, K. M. Murphy, D. K. Morrison, R. E. Lewis, J. McNeish, and A. S. Shaw. 2002. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niehof, M., S. Kubicka, L. Zender, M. P. Manns, and C. Trautwein. 2001. Autoregulation enables different pathways to control CCAAT/enhancer binding protein beta (C/EBP beta) transcription. J. Mol. Biol. 309:855-868. [DOI] [PubMed] [Google Scholar]
- 45.Ohmachi, M., C. E. Rocheleau, D. Church, E. Lambie, T. Schedl, and M. V. Sundaram. 2002. C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr. Biol. 12:427-433. [DOI] [PubMed] [Google Scholar]
- 46.Ory, S., M. Zhou, T. P. Conrads, T. D. Veenstra, and D. K. Morrison. 2003. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr. Biol. 13:1356-1364. [DOI] [PubMed] [Google Scholar]
- 47.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park, B.-H., L. Qiang, and S. R. Farmer. 2004. Phosphorylation of C/EBPβ at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol. Cell. Biol. 24:8671-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel, Y. M., and M. D. Lane. 2000. Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J. Biol. Chem. 275:17653-17660. [DOI] [PubMed] [Google Scholar]
- 50.Pearson, G., F. Robinson, T. B. Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 51.Perry, R. L., M. H. Parker, and M. A. Rudnicki. 2001. Activated MEK1 binds the nuclear MyoD transcriptional complex to repress transactivation. Mol. Cell 8:291-301. [DOI] [PubMed] [Google Scholar]
- 52.Piwien-Pilipuk, G., O. MacDougald, and J. Schwartz. 2002. Dual regulation of phosphorylation and dephosphorylation of C/EBPbeta modulate its transcriptional activation and DNA binding in response to growth hormone. J. Biol. Chem. 277:44557-44565. [DOI] [PubMed] [Google Scholar]
- 53.Prusty, D., B. H. Park, K. E. Davis, and S. R. Farmer. 2002. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 277:46226-46232. [DOI] [PubMed] [Google Scholar]
- 54.Qiu, Z., Y. Wei, N. Chen, M. Jiang, J. Wu, and K. Liao. 2001. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J. Biol. Chem. 276:11988-11995. [DOI] [PubMed] [Google Scholar]
- 55.Razidlo, G. L., R. L. Kortum, J. L. Haferbier, and R. E. Lewis. 2004. Phosphorylation regulates KSR1 stability, ERK activation, and cell proliferation. J. Biol. Chem. 279:47808-47814. [DOI] [PubMed] [Google Scholar]
- 56.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 16:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen, E. D., P. Sarraf, A. E. Troy, G. Bradwin, K. Moore, D. S. Milstone, B. M. Spiegelman, and R. M. Mortensen. 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4:611-617. [DOI] [PubMed] [Google Scholar]
- 58.Rosen, E. D., C. J. Walkey, P. Puigserver, and B. M. Spiegelman. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14:1293-1307. [PubMed] [Google Scholar]
- 59.Ross, S. E., N. Hemati, K. A. Longo, C. N. Bennett, P. C. Lucas, R. L. Erickson, and O. A. MacDougald. 2000. Inhibition of adipogenesis by Wnt signaling. Science 289:950-953. [DOI] [PubMed] [Google Scholar]
- 60.Ross, S. E., H. S. Radomska, B. Wu, P. Zhang, J. N. Winnay, L. Bajnok, W. S. Wright, F. Schaufele, D. G. Tenen, and O. A. MacDougald. 2004. Phosphorylation of C/EBPα inhibits granulopoiesis. Mol. Cell. Biol. 24:675-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy, F., G. Laberge, M. Douziech, D. Ferland-McCollough, and M. Therrien. 2002. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sale, E. M., P. G. Atkinson, and G. J. Sale. 1995. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 14:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 64.Sharp, L. L., D. A. Schwarz, C. M. Bott, C. J. Marshall, and S. M. Hedrick. 1997. The influence of the MAPK pathway on T cell lineage commitment. Immunity 7:609-618. [DOI] [PubMed] [Google Scholar]
- 65.Stephens, J. M., R. F. Morrison, Z. Wu, and S. R. Farmer. 1999. PPARgamma ligand-dependent induction of STAT1, STAT5A, and STAT5B during adipogenesis. Biochem. Biophys. Res. Commun. 262:216-222. [DOI] [PubMed] [Google Scholar]
- 66.Stewart, S., M. Sundaram, Y. Zhang, J. Lee, M. Han, and K. L. Guan. 1999. Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol. Cell. Biol. 19:5523-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Student, A. K., R. Y. Hsu, and M. D. Lane. 1980. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 255:4745-4750. [PubMed] [Google Scholar]
- 68.Sundaram, M., and M. Han. 1995. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell 83:889-901. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka, T., N. Yoshida, T. Kishimoto, and S. Akira. 1997. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 16:7432-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang, Q. Q., T. C. Otto, and M. D. Lane. 2003. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 100:850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang, Q. Q., T. C. Otto, and M. D. Lane. 2003. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 100:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teis, D., W. Wunderlich, and L. A. Huber. 2002. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3:803-814. [DOI] [PubMed] [Google Scholar]
- 73.Therrien, M., H. C. Chang, N. M. Solomon, F. D. Karim, D. A. Wassarman, and G. M. Rubin. 1995. KSR, a novel protein kinase required for RAS signal transduction. Cell 83:879-888. [DOI] [PubMed] [Google Scholar]
- 74.Therrien, M., N. R. Michaud, G. M. Rubin, and D. K. Morrison. 1996. KSR modulates signal propagation within the MAPK cascade. Genes Dev. 10:2684-2695. [DOI] [PubMed] [Google Scholar]
- 75.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Traverse, S., N. Gomez, H. Paterson, C. Marshall, and P. Cohen. 1992. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uhlik, M. T., A. N. Abell, N. L. Johnson, W. Sun, B. D. Cuevas, K. E. Lobel-Rice, E. A. Horne, M. L. Dell'Acqua, and G. L. Johnson. 2003. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 5:1104-1110. [DOI] [PubMed] [Google Scholar]
- 78.Wang, X., and G. P. Studzinski. 2004. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J. Cell. Physiol. 198:333-342. [DOI] [PubMed] [Google Scholar]
- 79.White, M. A., C. Nicolette, A. Minden, A. Polverino, L. Van Aelst, M. Karin, and M. H. Wigler. 1995. Multiple Ras functions can contribute to mammalian cell transformation. Cell 80:533-541. [DOI] [PubMed] [Google Scholar]
- 80.Whitmarsh, A. J., J. Cavanagh, C. Tournier, J. Yasuda, and R. J. Davis. 1998. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281:1671-1674. [DOI] [PubMed] [Google Scholar]
- 81.Whitmarsh, A. J., C. Y. Kuan, N. J. Kennedy, N. Kelkar, T. F. Haydar, J. P. Mordes, M. Appel, A. A. Rossini, S. N. Jones, R. A. Flavell, P. Rakic, and R. J. Davis. 2001. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 15:2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu, Z., N. L. Bucher, and S. R. Farmer. 1996. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 16:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu, Z., P. Puigserver, and B. M. Spiegelman. 1999. Transcriptional activation of adipogenesis. Curr. Opin. Cell Biol. 11:689-694. [DOI] [PubMed] [Google Scholar]
- 84.Wu, Z., E. D. Rosen, R. Brun, S. Hauser, G. Adelmant, A. E. Troy, C. McKeon, G. J. Darlington, and B. M. Spiegelman. 1999. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3:151-158. [DOI] [PubMed] [Google Scholar]
- 85.Wu, Z., Y. Xie, N. L. Bucher, and S. R. Farmer. 1995. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev. 9:2350-2363. [DOI] [PubMed] [Google Scholar]
- 86.Yang, L., and N. E. Baker. 2003. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell 4:359-369. [DOI] [PubMed] [Google Scholar]
- 87.Yao, Y., W. Li, J. Wu, U. A. Germann, M. S. Su, K. Kuida, and D. M. Boucher. 2003. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl. Acad. Sci. USA 100:12759-12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang, B., J. Berger, G. Zhou, A. Elbrecht, S. Biswas, S. White-Carrington, D. Szalkowski, and D. E. Moller. 1996. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 271:31771-31774. [DOI] [PubMed] [Google Scholar]
- 89.Zhang, J. W., D. J. Klemm, C. Vinson, and M. D. Lane. 2004. Role of CREB in transcriptional regulation of CCAAT/enhancer binding protein β gene during adipogenesis. J. Biol. Chem. 279:4471-4478. [DOI] [PubMed] [Google Scholar]
- 90.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]