Abstract
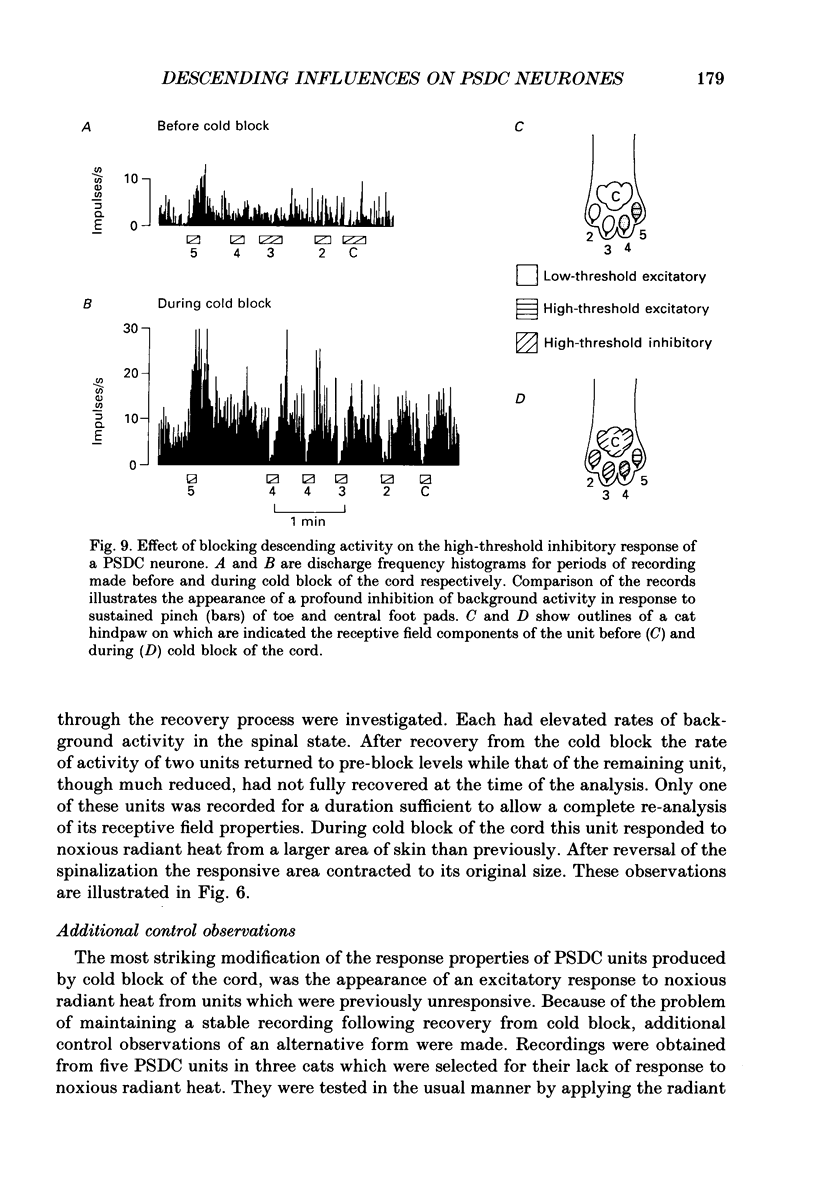
1. The influence of activity in descending systems on the cutaneous receptive field properties of postsynaptic dorsal column (PSDC) neurones has been investigated in chloralose-anaesthetized cats. The main aim of the study was to determine whether the receptive field boundaries of PSDC neurones are under the control of systems descending from the brain. 2. Single-unit recordings were made from the ascending axons of PSDC units in the dorsal columns. Receptive fields were analysed using light tactile and noxious mechanical and thermal stimuli, both before and during a reversible block of spinal conduction produced by cooling the cord rostral of the recording site. 3. The light tactile excitatory fields of PSDC neurones were largely unaffected by the cold-block procedure. 4. In contrast, both the sensitivity of PSDC neurones to noxious stimuli and the area of skin from which they could be effectively excited by such stimuli were found to be profoundly modified by interruption of descending activity. Two-thirds of the units excited by noxious pinch responded more vigorously in the cold-blocked state and one-half from an expanded area of skin. Responses to noxious radiant heat were similarly modified. 5. Inhibition evoked in PSDC neurones, whether by light tactile or noxious stimuli, involved predominantly segmental mechanisms since it remained effective in the cold-blocked state. 6. It is concluded that neurones of the PSDC system are amongst those dorsal horn neurones with receptive field geometries which may be modified by activity in descending systems.
Full text
PDF



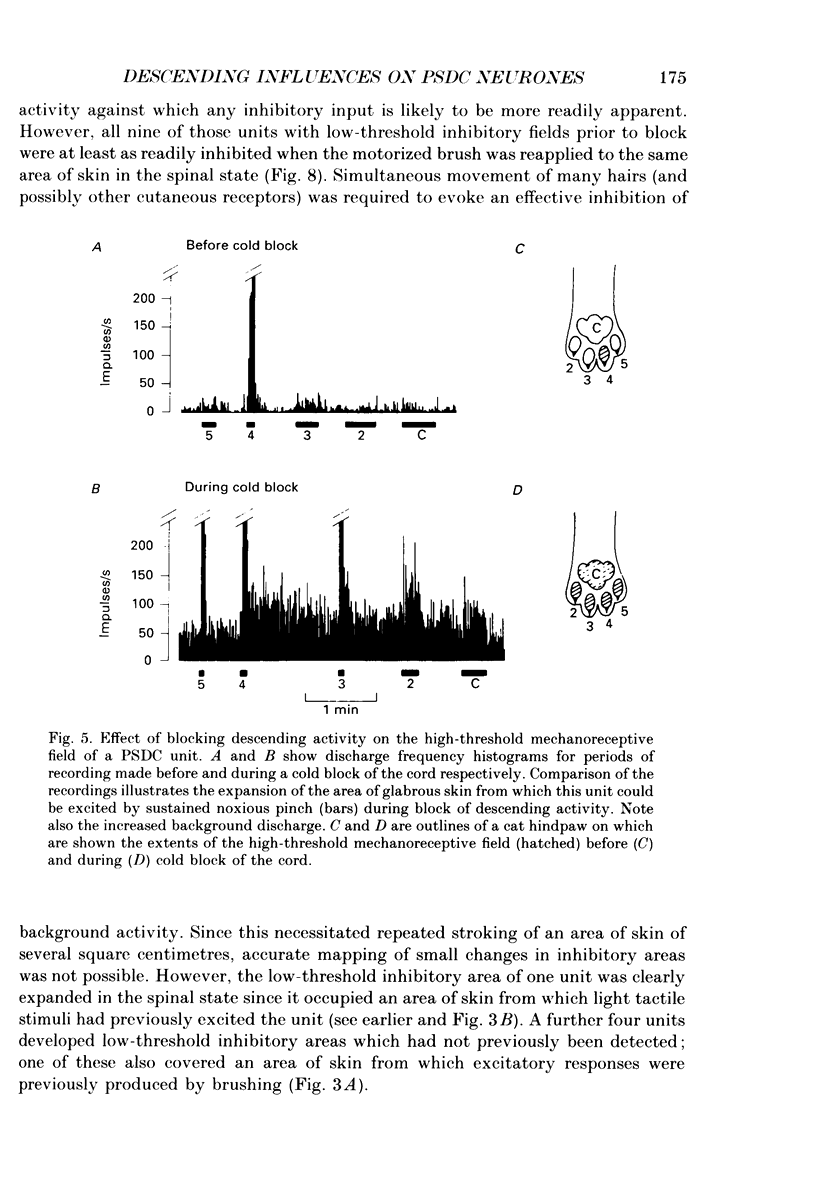
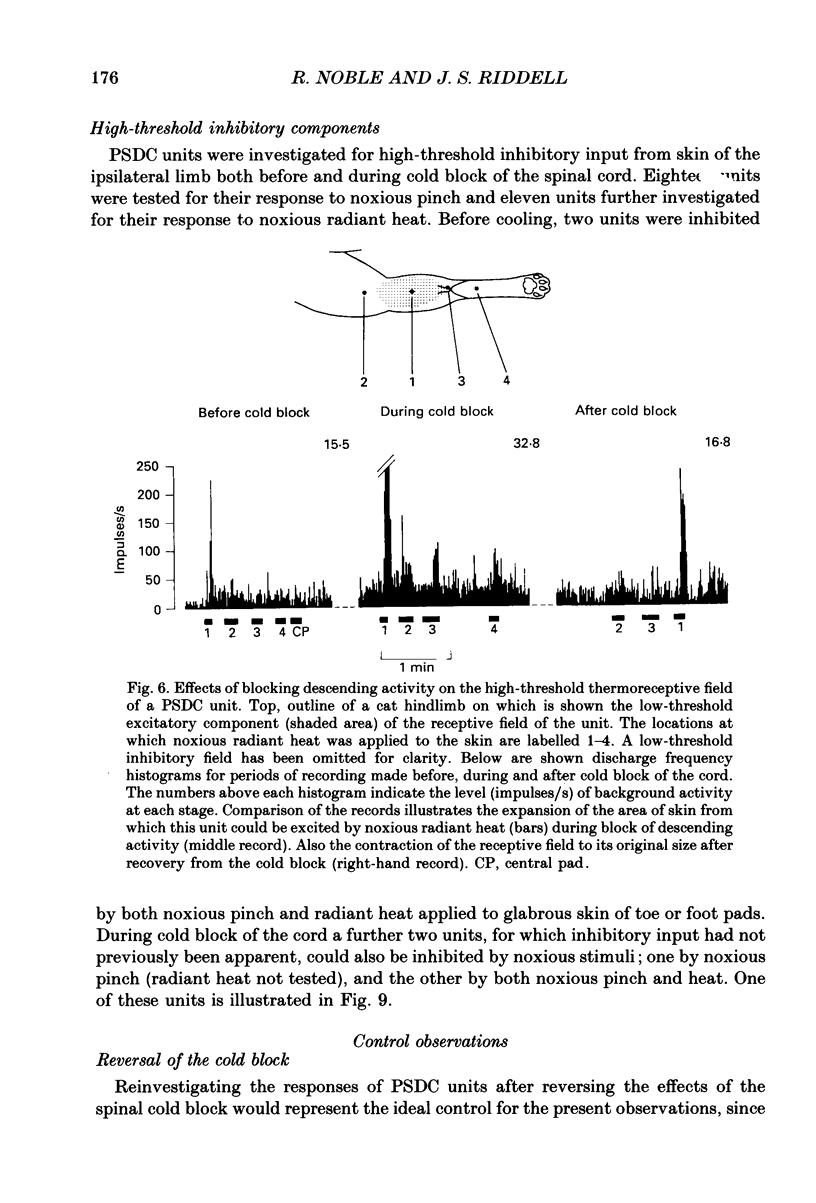
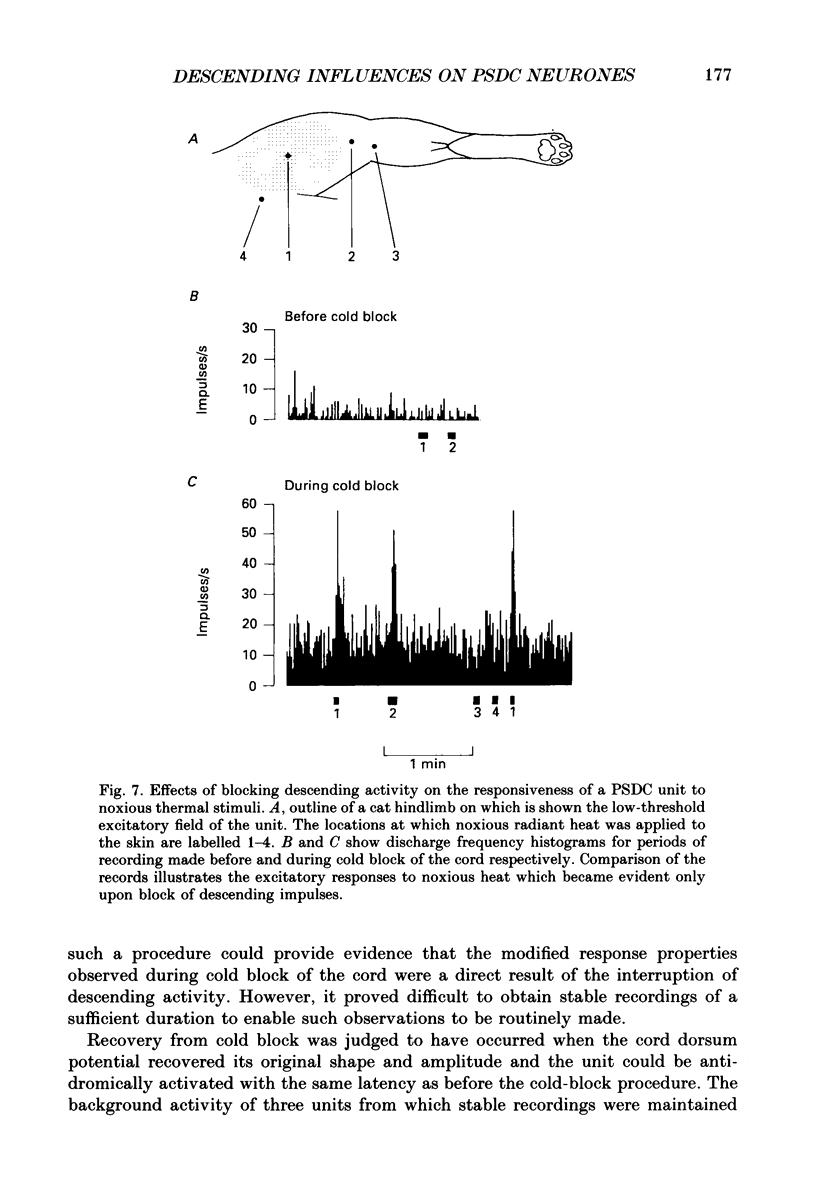
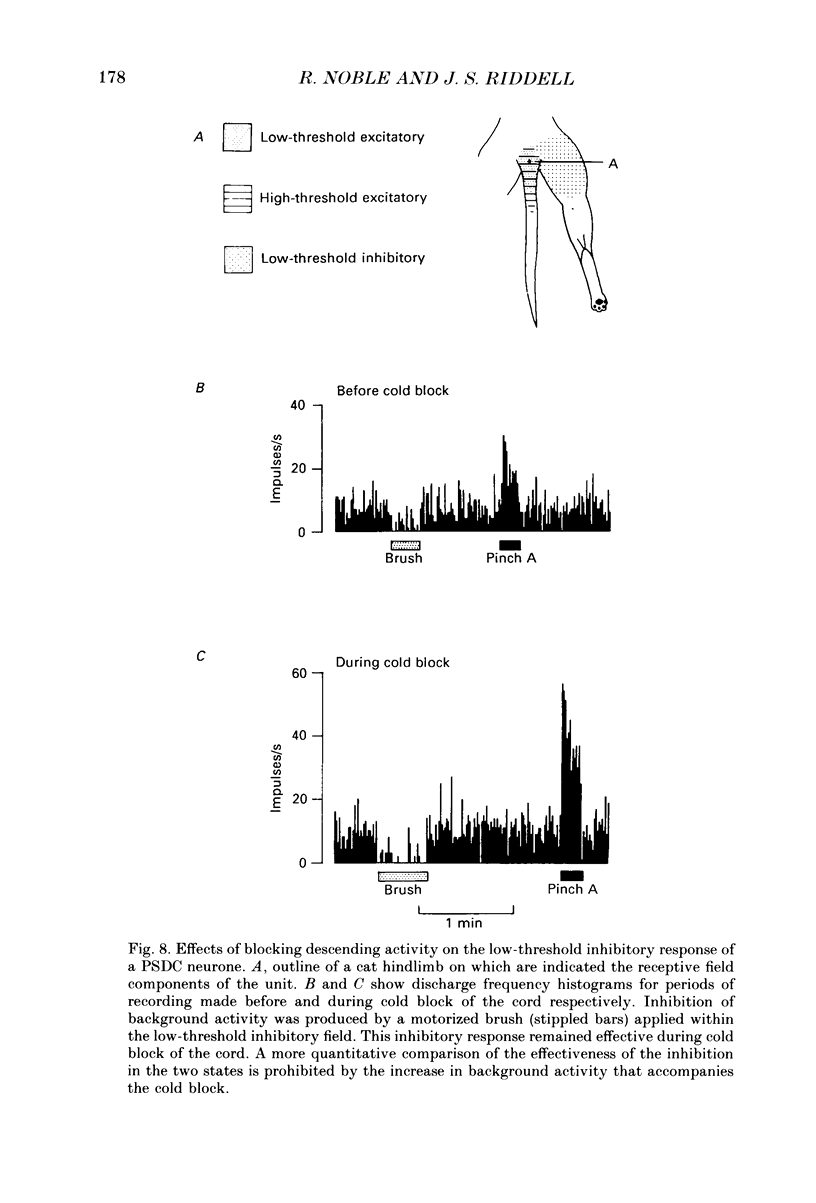




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R., Riddell J. S. Relations between spinocervical and post-synaptic dorsal column neurones in the cat. J Physiol. 1986 Dec;381:333–349. doi: 10.1113/jphysiol.1986.sp016330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977 May;267(2):537–558. doi: 10.1113/jphysiol.1977.sp011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. W. The effects of decerebration and destruction of nucleus raphe magnus, periaqueductal grey matter and brainstem lateral reticular formation on the depression due to surgical trauma of the jaw-opening reflex evoked by tooth-pulp stimulation in the cat. Brain Res. 1985 Apr 22;332(2):231–236. doi: 10.1016/0006-8993(85)90592-x. [DOI] [PubMed] [Google Scholar]
- Croze S., Duclaux R., Kenshalo D. R. The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol. 1976 Dec;263(3):539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A. M. Cells of the dorsal column nuclei projecting down into the spinal cord. J Physiol. 1971 Dec;219(2):29P–30P. [PubMed] [Google Scholar]
- Dickhaus H., Pauser G., Zimmermann M. Tonic descending inhibition affects intensity coding of nociceptive responses of spinal dorsal horn neurones in the cat. Pain. 1985 Oct;23(2):145–158. doi: 10.1016/0304-3959(85)90056-9. [DOI] [PubMed] [Google Scholar]
- Enevoldson T. P., Gordon G. Spinally projecting neurons in the dorsal column nuclei: distribution, dendritic trees and axonal projections. A retrograde HRP study in the cat. Exp Brain Res. 1984;54(3):538–550. doi: 10.1007/BF00235479. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta Physiol Scand. 1968 Jan-Feb;72(1):123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977 Feb;265(2):549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E., KERR D. I. Central influences on spinal afferent conduction. J Neurophysiol. 1954 May;17(3):295–307. doi: 10.1152/jn.1954.17.3.295. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Duggan A. W., Morton C. R., Johnson S. M. The location of brainstem neurones tonically inhibiting dorsal horn neurones of the cat. Brain Res. 1982 Jul 29;244(2):215–222. doi: 10.1016/0006-8993(82)90080-4. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Iggo A., Zimmermann M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975 Jun;1(2):147–165. doi: 10.1016/0304-3959(75)90099-8. [DOI] [PubMed] [Google Scholar]
- Hayes R. L., Dubner R., Hoffman D. S. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. J Neurophysiol. 1981 Sep;46(3):428–443. doi: 10.1152/jn.1981.46.3.428. [DOI] [PubMed] [Google Scholar]
- Hillman P., Wall P. D. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9(4):284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977 Nov;40(6):1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Morton C. R., Johnson S. M., Duggan A. W. Lateral reticular regions and the descending control of dorsal horn neurones of the cat: selective inhibition by electrical stimulation. Brain Res. 1983 Sep 19;275(1):13–21. doi: 10.1016/0006-8993(83)90413-4. [DOI] [PubMed] [Google Scholar]
- Noble R., Riddell J. S. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol. 1988 Feb;396:497–513. doi: 10.1113/jphysiol.1988.sp016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D. On the relation of injury to pain. The John J. Bonica lecture. Pain. 1979 Jun;6(3):253–264. doi: 10.1016/0304-3959(79)90047-2. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]


