Abstract
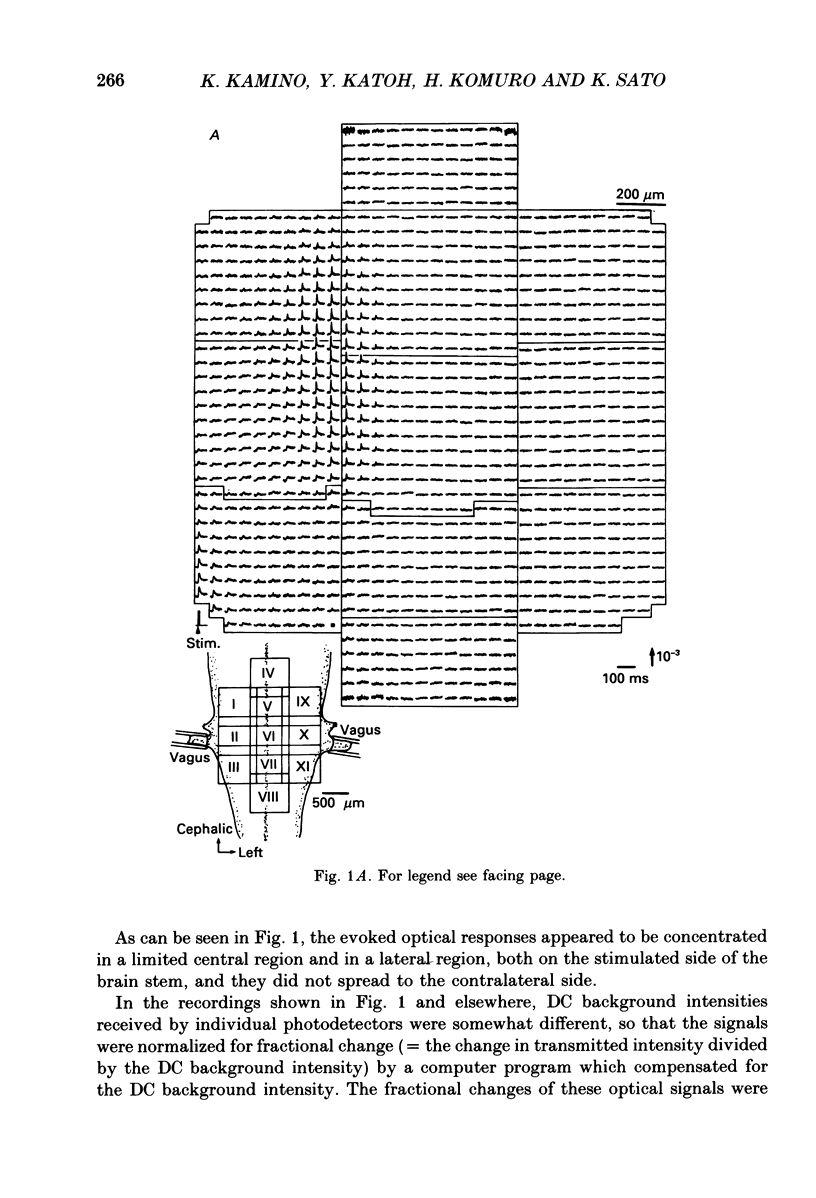
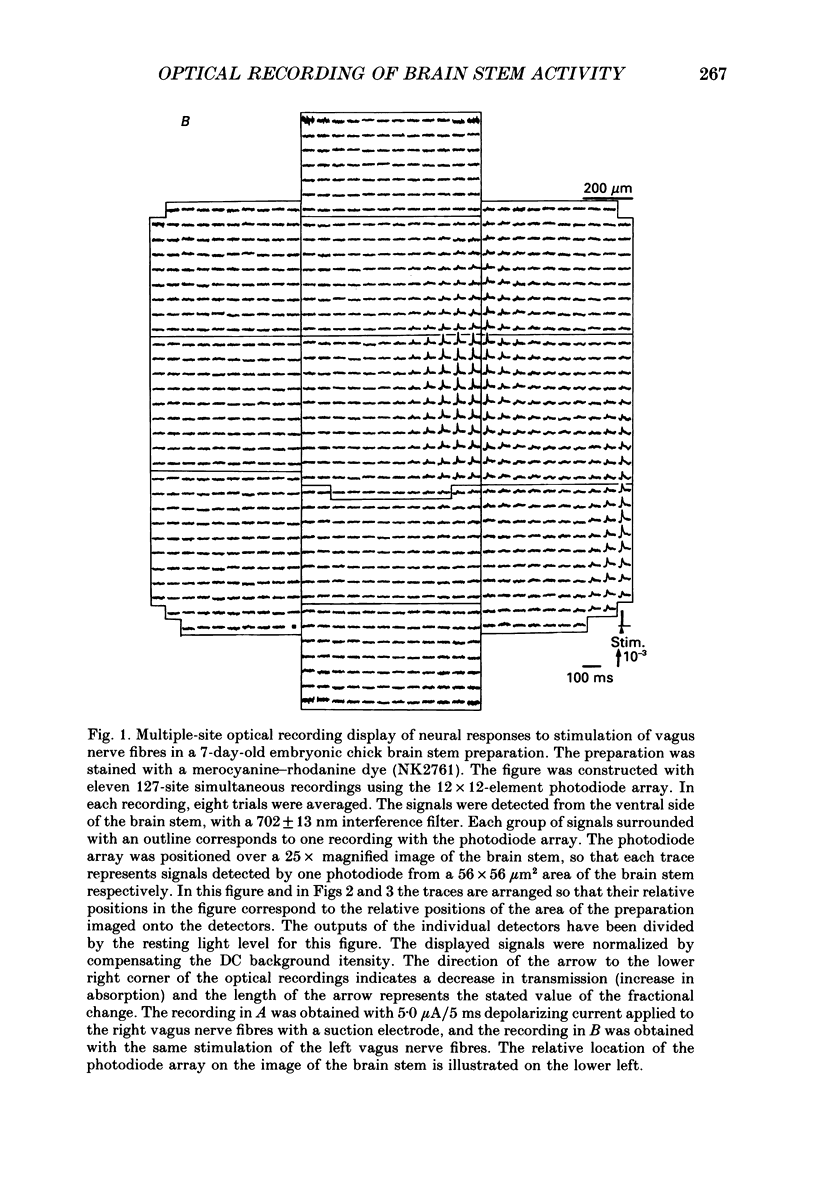
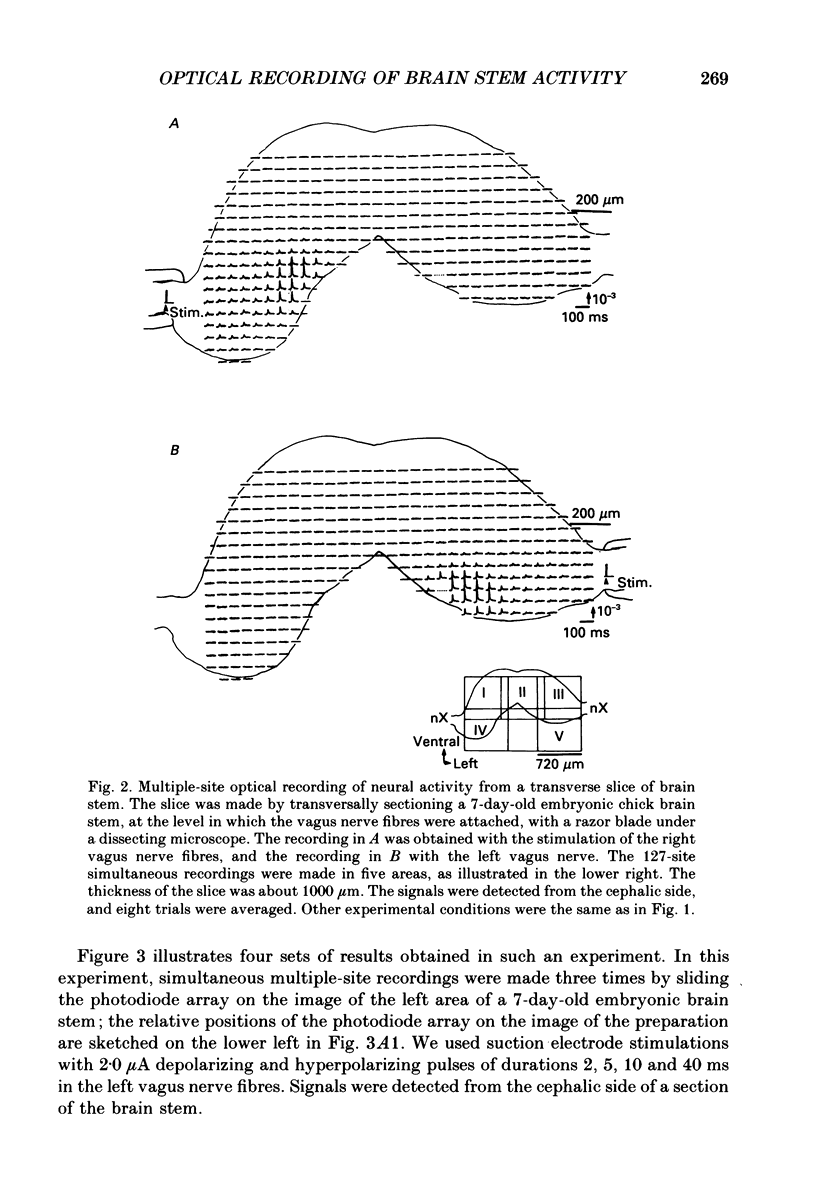
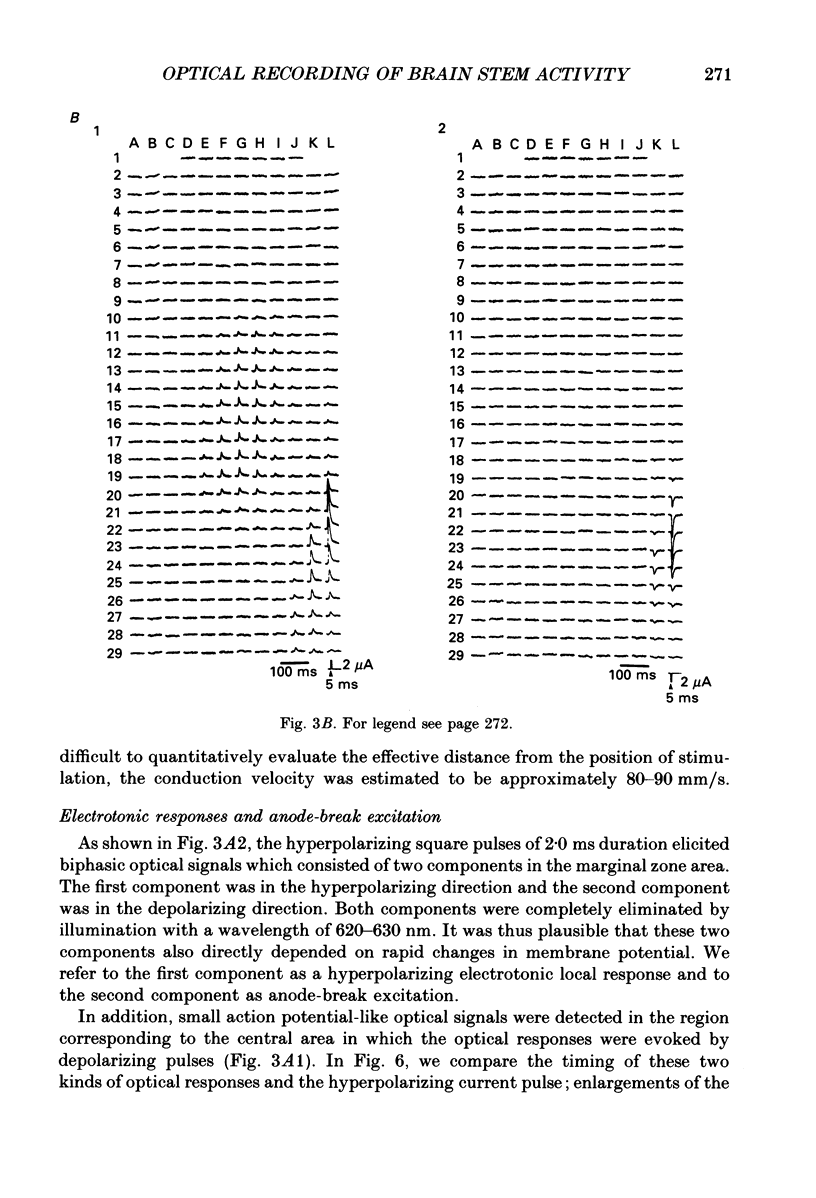
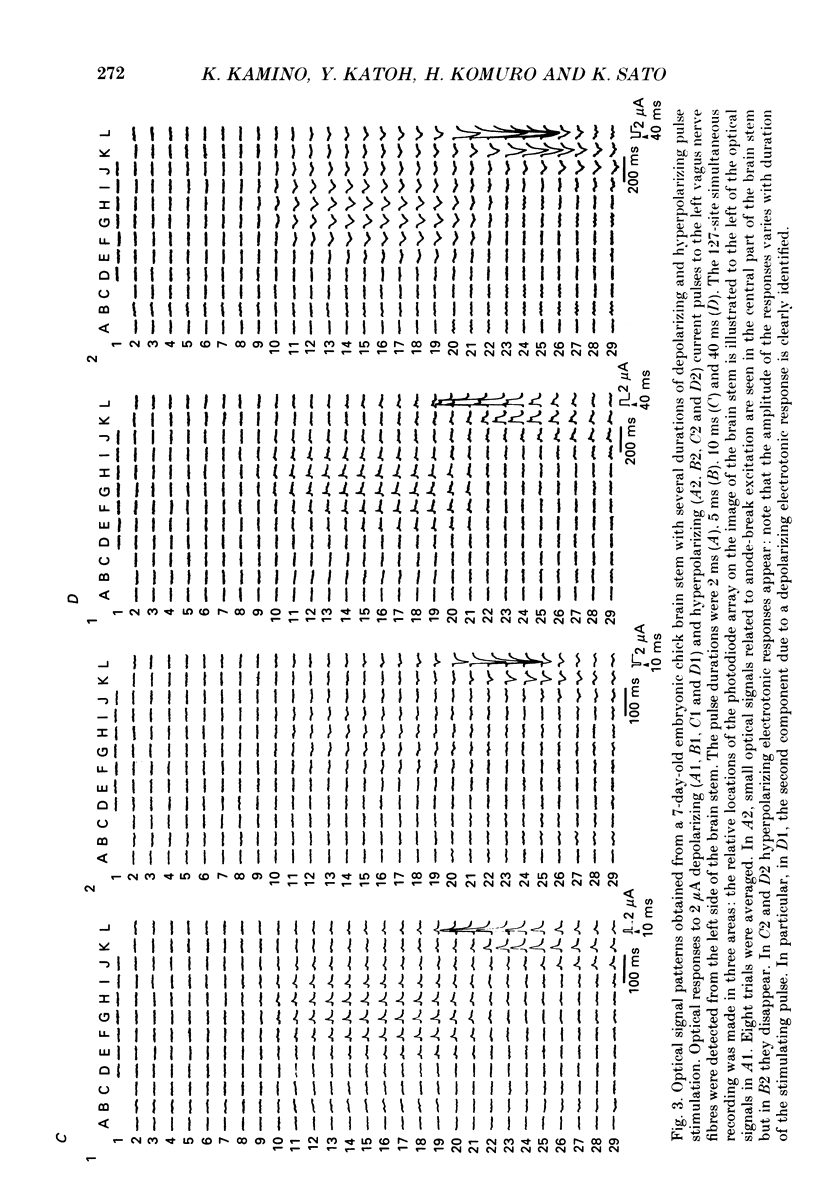
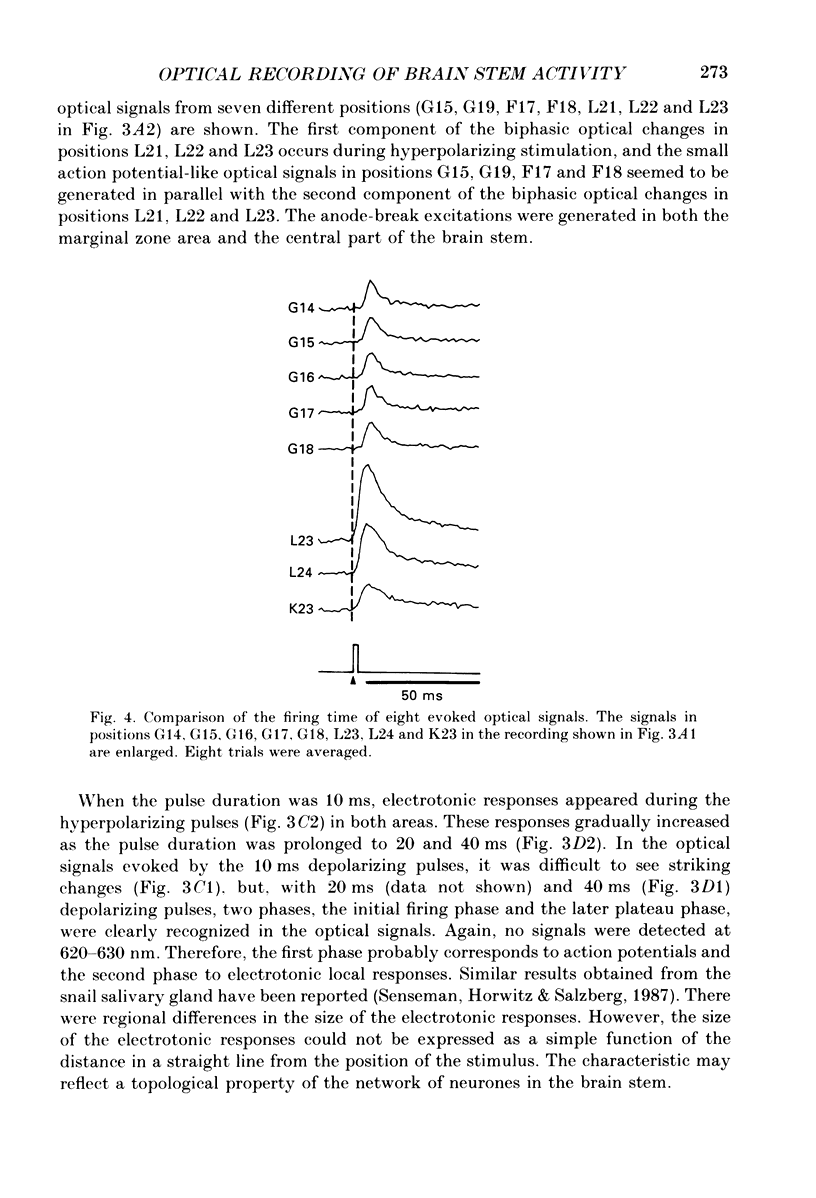
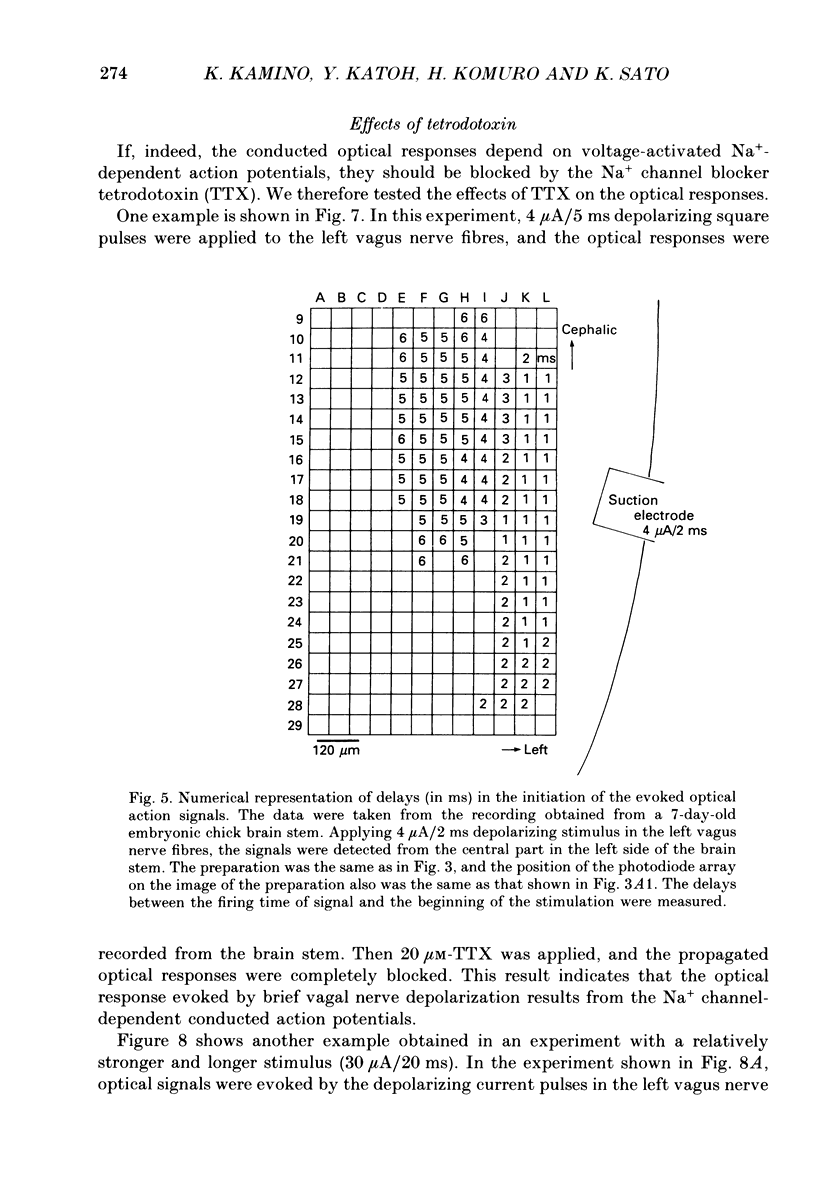
1. Electrical activity in the embryonic chick brain stem has been monitored optically. The vagus-brain stem preparations isolated from 7-day-old chick embryos were stained with voltage-sensitive merocyanine-rhodanine dyes. 2. Voltage-related optical absorption signals evoked by vagus nerve stimulation with depolarizing and hyperpolarizing pulses using a suction electrode were recorded simultaneously from 127 adjacent loci in the brain stem using a 12 x 12-element photodiode array. 3. The optical signals evoked by the stimulation appeared to be concentrated longitudinally in the central region and in the lateral region, both on the stimulated side of the brain stem, and they did not spread to the opposite side. In addition, the evoked optical responses were detected from small areas on the dorsal surface of the stimulated side, in experiments using transverse slices of brain stem. 4. The optical action potential signals evoked by the brief depolarizing stimulus were conducted slowly and were blocked completely by tetrodotoxin. With relatively long-duration depolarizing and hyperpolarizing stimulations, electrotonic responses were recorded. 5. When 2 microA/2 ms hyperpolarizing pulse stimulations were applied, anode-break excitation signals were detected, and these signals were also blocked by tetrodotoxin. 6. On the basis of the data obtained from these experiments, we constructed maps of the electrical response area and demonstrated the spatial pattern of the vagus dorsal nucleus in the 7-day-old embryonic chick brain stem.
Full text
PDF



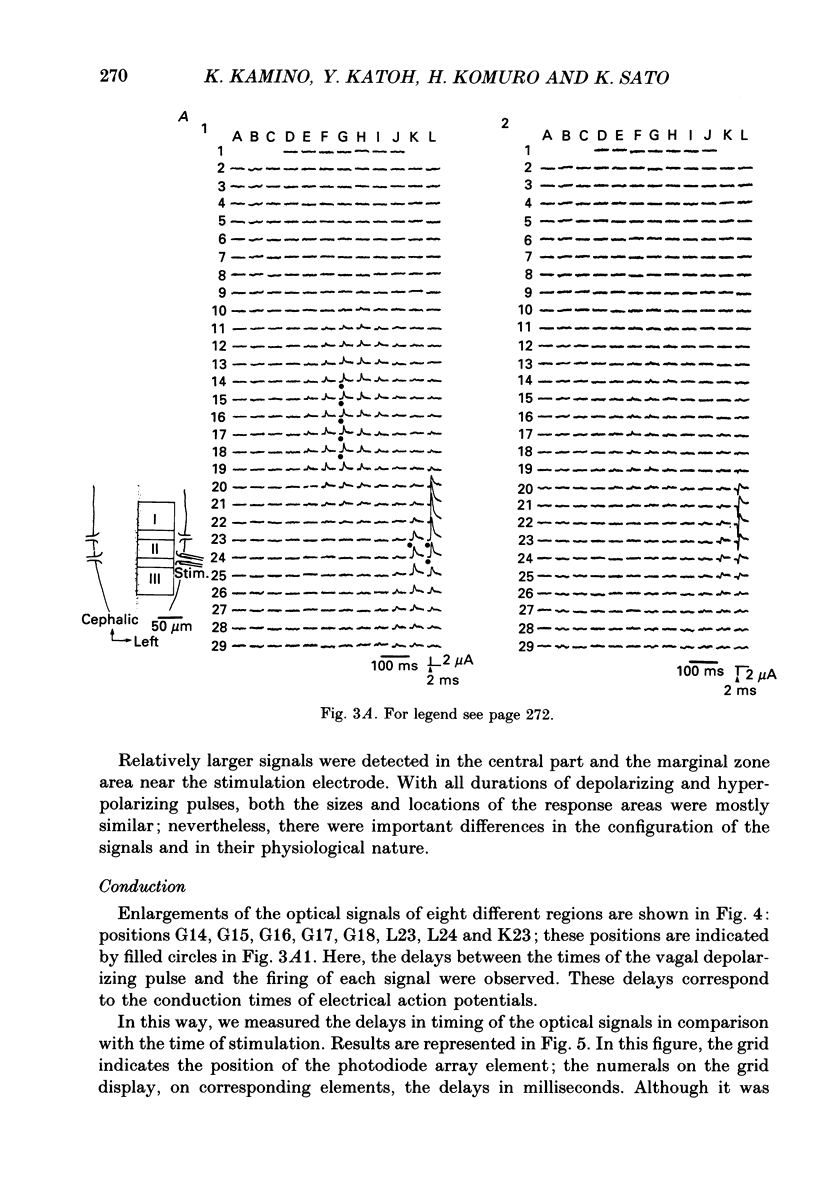





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen D. H., Schnall A. M., Macdonald R. L., Pitts L. H. Medullary cells of origin of vagal cardioinhibitory fibers in the pigeon. I. Anatomical studies of peripheral vagus nerve and the dorsal motor nucleus. J Comp Neurol. 1970 Nov;140(3):299–320. doi: 10.1002/cne.901400305. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Freedman J. C., Laris P. C. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl. 1981;12:177–246. doi: 10.1016/b978-0-12-364373-5.50015-9. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol. 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Anglister L., Freeman J. A., Hildesheim R., Manker A. Real-time optical imaging of naturally evoked electrical activity in intact frog brain. 1984 Apr 26-May 2Nature. 308(5962):848–850. doi: 10.1038/308848a0. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Farber I. C. Optical recording of calcium action potentials from growth cones of cultured neurons with a laser microbeam. Science. 1981 Jun 5;212(4499):1164–1167. doi: 10.1126/science.7233210. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Heaton M. B., Moody S. A. Early development and migration of the trigeminal motor nucleus in the chick embryo. J Comp Neurol. 1980 Jan 1;189(1):61–99. doi: 10.1002/cne.901890105. [DOI] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T. Mapping of early development of electrical activity in the embryonic chick heart using multiple-site optical recording. J Physiol. 1987 Feb;383:711–728. doi: 10.1113/jphysiol.1987.sp016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T., Yada T. Early events in development of electrical activity and contraction in embryonic rat heart assessed by optical recording. J Physiol. 1985 Dec;369:209–227. doi: 10.1113/jphysiol.1985.sp015897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T., Yada T. Optical studies of excitation-contraction coupling in the early embryonic chick heart. J Physiol. 1985 Sep;366:89–106. doi: 10.1113/jphysiol.1985.sp015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H., Hackett J. T., Rubel E. W. Organization and development of brain stem auditory nuclei in the chick: ontogeny of postsynaptic responses. J Comp Neurol. 1982 Sep 1;210(1):80–86. doi: 10.1002/cne.902100109. [DOI] [PubMed] [Google Scholar]
- Jackson H., Rubel E. W. Ontogeny of behavioral responsiveness to sound in the chick embryo as indicated by electrical recordings of motility. J Comp Physiol Psychol. 1978 Aug;92(4):682–696. doi: 10.1037/h0077496. [DOI] [PubMed] [Google Scholar]
- Kamino K., Hirota A., Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981 Apr 16;290(5807):595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- Kamino K., Komuro H., Sakai T., Hirota A. Functional pacemaking area in the early embryonic chick heart assessed by simultaneous multiple-site optical recording of spontaneous action potentials. J Gen Physiol. 1988 Apr;91(4):573–591. doi: 10.1085/jgp.91.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K., Komuro H., Sakai T. Regional gradient of pacemaker activity in the early embryonic chick heart monitored by multisite optical recording. J Physiol. 1988 Aug;402:301–314. doi: 10.1113/jphysiol.1988.sp017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. S., Senseman D. M., Cohen L. B. Odor-elicited activity monitored simultaneously from 124 regions of the salamander olfactory bulb using a voltage-sensitive dye. Brain Res. 1987 Aug 25;418(2):255–261. doi: 10.1016/0006-8993(87)90093-x. [DOI] [PubMed] [Google Scholar]
- Komuro H., Hirota A., Yada T., Sakai T., Fujii S., Kamino K. Effects of calcium on electrical propagation in early embryonic precontractile heart as revealed by multiple-site optical recording of action potentials. J Gen Physiol. 1985 Mar;85(3):365–382. doi: 10.1085/jgp.85.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H., Sakai T., Hirota A., Kamino K. Conduction pattern of excitation in the amphibian atrium assessed by multiple-site optical recording of action potentials. Jpn J Physiol. 1986;36(1):123–137. doi: 10.2170/jjphysiol.36.123. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Obaid A. L., Salzberg B. M. Optical recording of electrical activity from parallel fibres and other cell types in skate cerebellar slices in vitro. J Physiol. 1987 Dec;393:681–702. doi: 10.1113/jphysiol.1987.sp016848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. A., Zecević D., Cohen L. B. Simultaneous optical recording of activity from many neurons during feeding in Navanax. J Neurosci. 1987 Mar;7(3):649–661. doi: 10.1523/JNEUROSCI.07-03-00649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A. L., Orkand R. K., Gainer H., Salzberg B. M. Active calcium responses recorded optically from nerve terminals of the frog neurohypophysis. J Gen Physiol. 1985 Apr;85(4):481–489. doi: 10.1085/jgp.85.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H. S., Cohen L. B., Grinvald A. Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci. 1985 Jul;5(7):1886–1895. doi: 10.1523/JNEUROSCI.05-07-01886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H. S., Cohen L. B. Optical monitoring of activity from many areas of the in vitro and in vivo salamander olfactory bulb: a new method for studying functional organization in the vertebrate central nervous system. J Neurosci. 1983 Nov;3(11):2251–2262. doi: 10.1523/JNEUROSCI.03-11-02251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Sakai T., Hirota A., Komuro H., Fujii S., Kamino K. Optical recording of membrane potential responses from early embryonic chick ganglia using voltage-sensitive dyes. Brain Res. 1985 Jan;349(1-2):39–51. doi: 10.1016/0165-3806(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Salama G., Lombardi R., Elson J. Maps of optical action potentials and NADH fluorescence in intact working hearts. Am J Physiol. 1987 Feb;252(2 Pt 2):H384–H394. doi: 10.1152/ajpheart.1987.252.2.H384. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Gainer H. Large and rapid changes in light scattering accompany secretion by nerve terminals in the mammalian neurohypophysis. J Gen Physiol. 1985 Sep;86(3):395–411. doi: 10.1085/jgp.86.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Senseman D. M., Horwitz I. S., Salzberg B. M. MSORTV imaging of electrotonic conduction in a syncitium: optical recording of polarization spread in a simple salivary gland. J Exp Zool. 1987 Oct;244(1):79–88. doi: 10.1002/jez.1402440110. [DOI] [PubMed] [Google Scholar]
- Senseman D. M., Shimizu H., Horwitz I. S., Salzberg B. M. Multiple-site optical recording of membrane potential from a salivary gland. Interaction of synaptic and electrotonic excitation. J Gen Physiol. 1983 Jun;81(6):887–908. doi: 10.1085/jgp.81.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. R., Rubel E. W. Embryogenesis of arborization pattern and topography of individual axons in N. laminaris of the chicken brain stem. J Comp Neurol. 1986 Dec 22;254(4):425–459. doi: 10.1002/cne.902540402. [DOI] [PubMed] [Google Scholar]


