Abstract
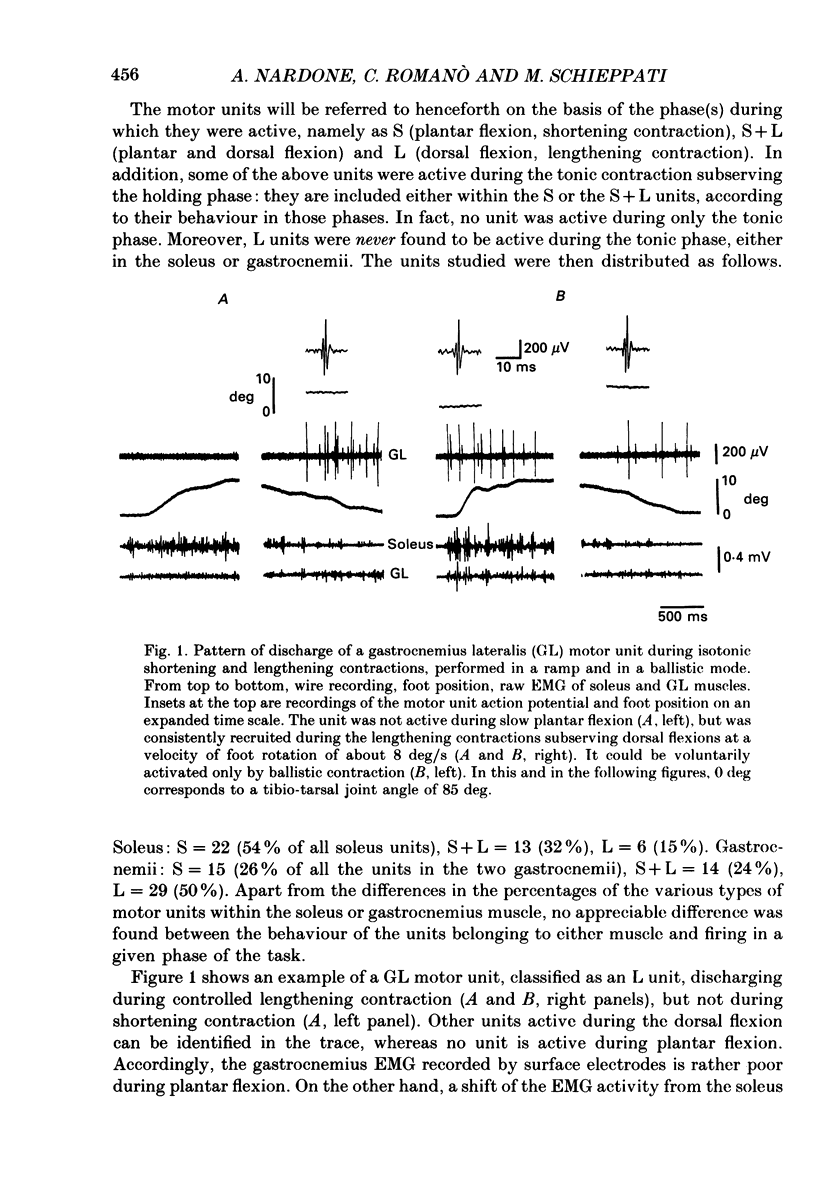
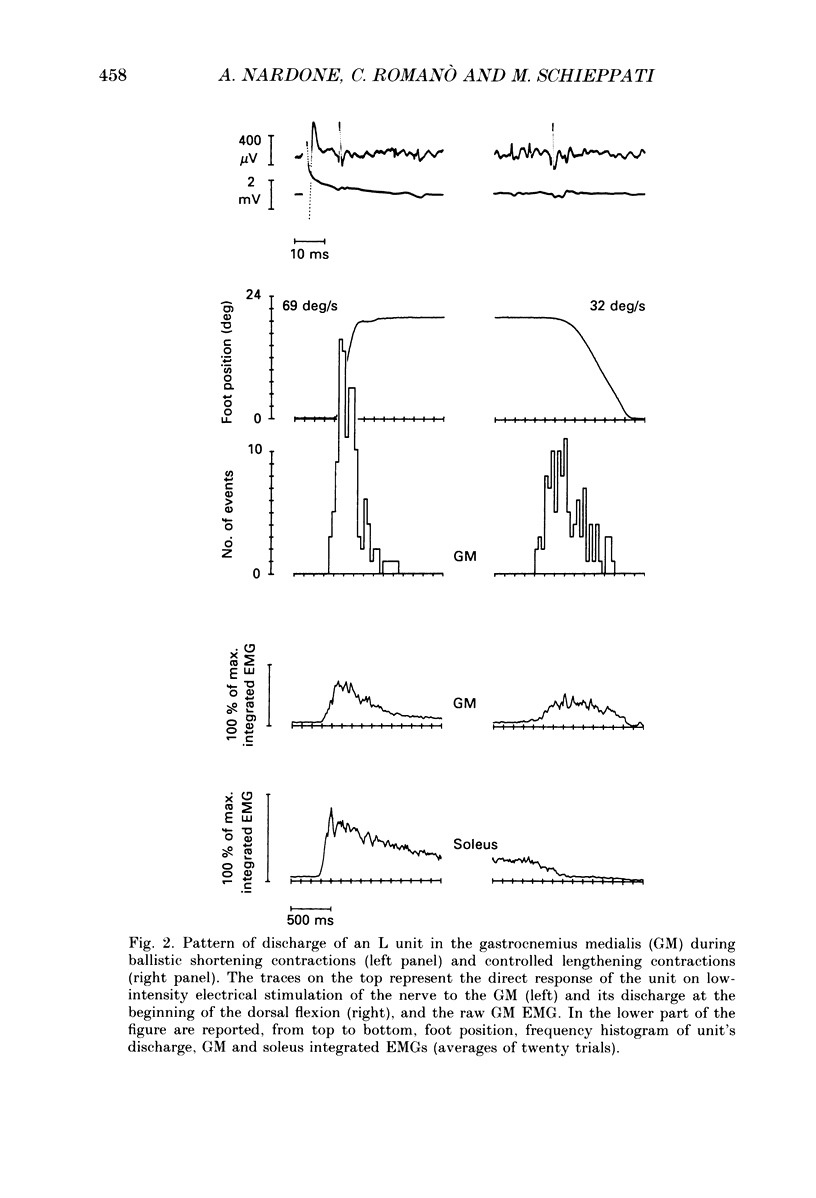
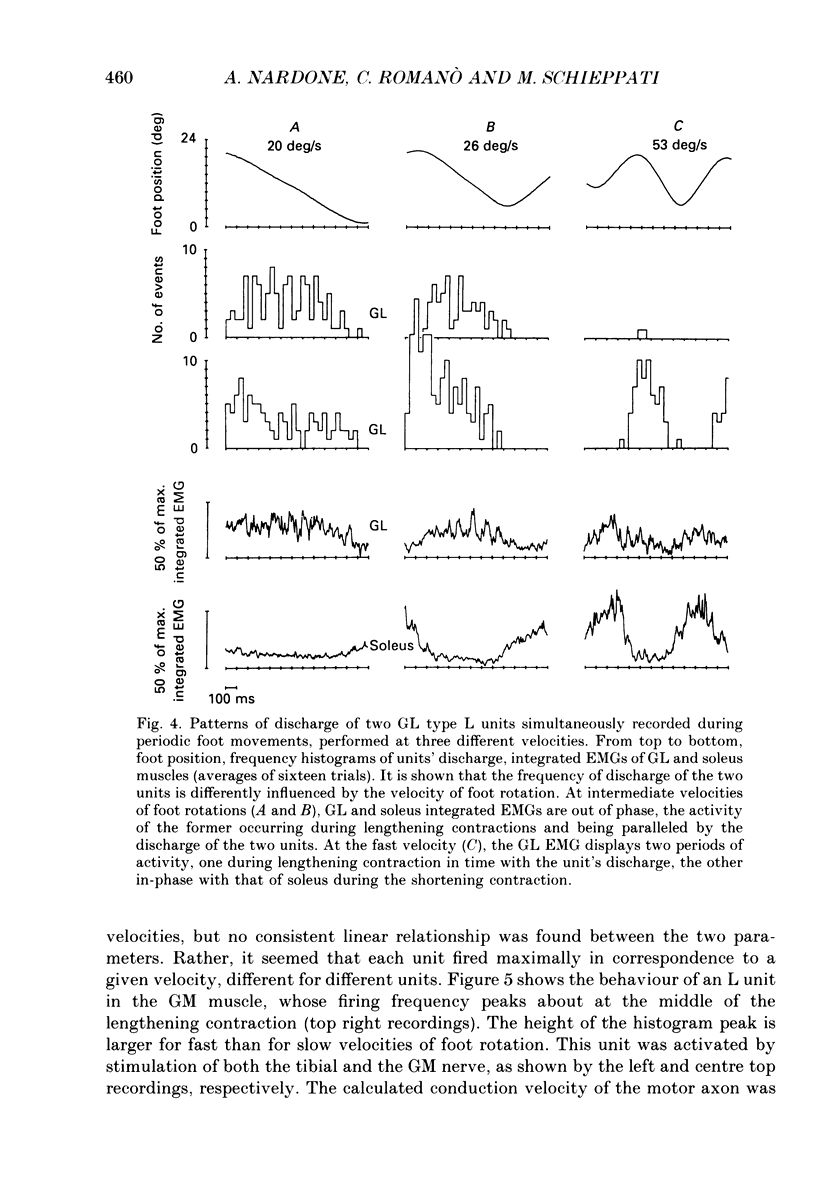
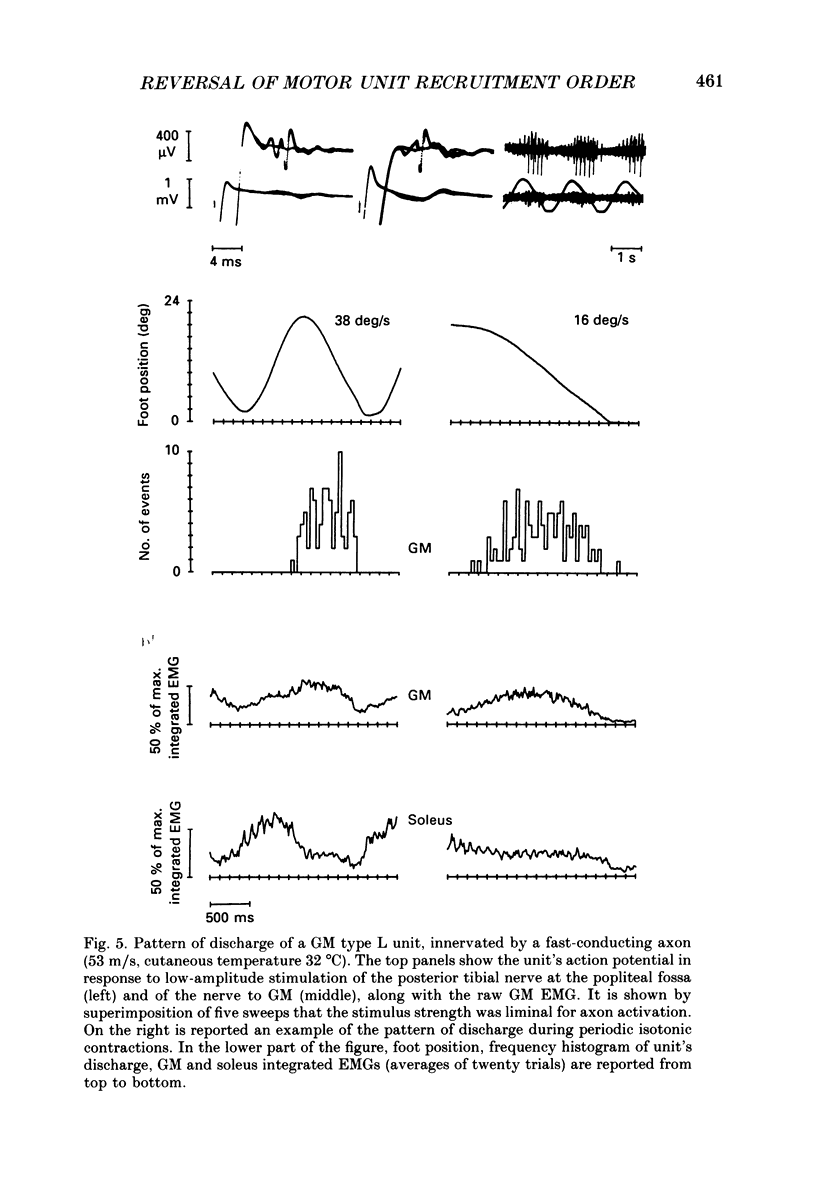
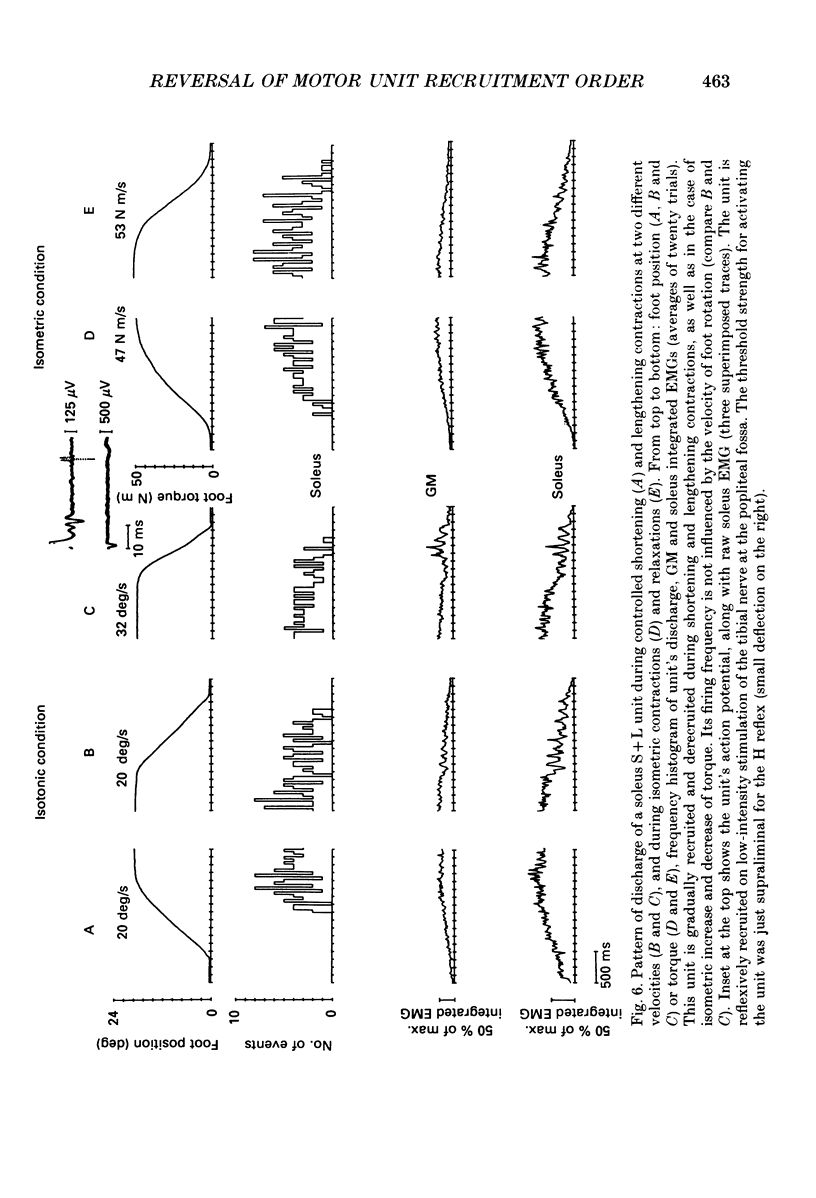
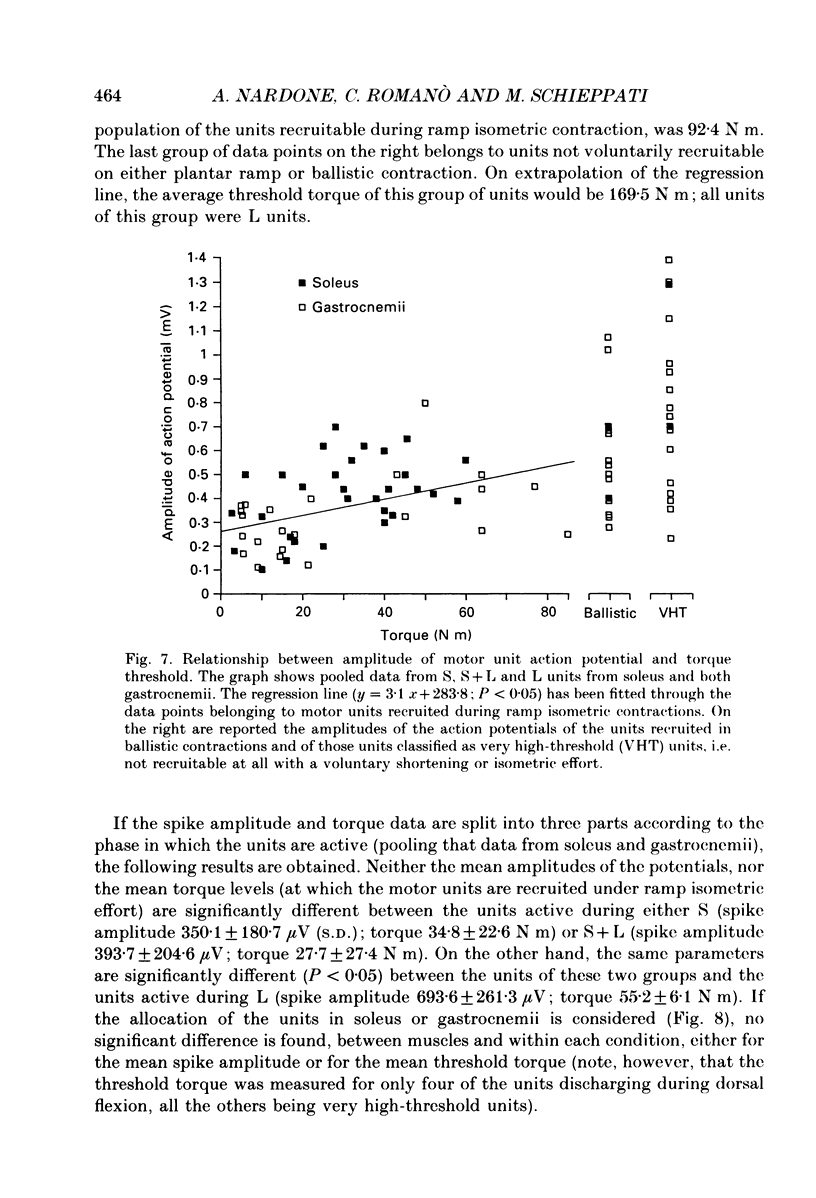
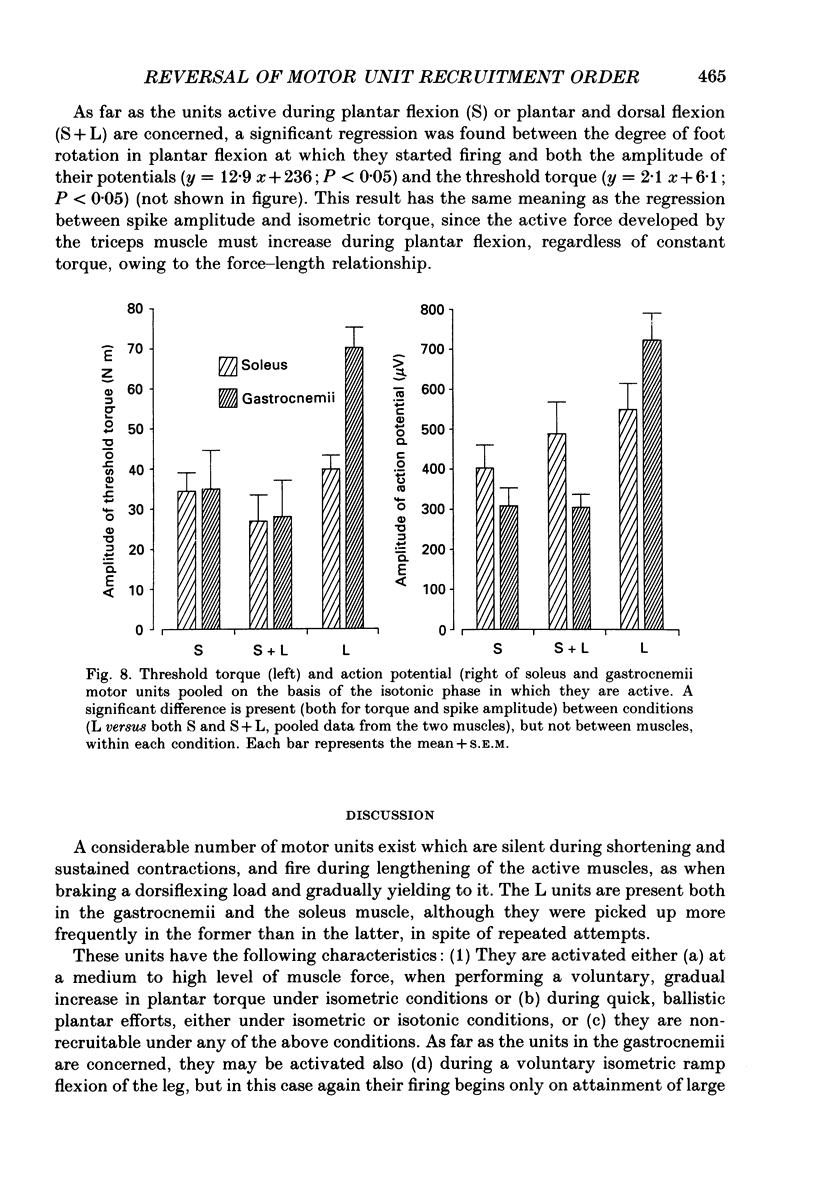
1. We have investigated the possibility that voluntary muscle lengthening contractions can be performed by selective recruitment of fast-twitch motor units, accompanied by derecruitment of slow-twitch motor units. 2. The behaviour of motor units in soleus, gastrocnemius lateralis and gastrocnemius medialis muscles was studied during (a) controlled isotonic plantar flexion against a constant load (shortening contraction, S), maintained plantar flexion, or dorsal flexion resisting the load and gradually yielding to it (lengthening contraction, L), (b) isometric increasing or decreasing plantar torque accomplished by graded contraction or relaxation of the triceps surae muscles, (c) isometric or isotonic ballistic contractions, and (d) periodic, quasi-sinusoidal isotonic contractions at different velocities. The above tasks were performed under visual control of foot position, without activation of antagonist muscles. The motor units discharging during foot rotation were grouped on the basis of the phase(s) during which they were active as S, S + L and L. The units were also characterized according to both the level of isometric ramp plantar torque at which they were first recruited and the amplitude of their action potential. 3. S units were never active during dorsal flexion; some of them were active during the sustained contraction between plantar and dorsal flexion. Most S + L units were active also during the maintenance phase and were slowly derecruited during lengthening; their behaviour during foot rotations was similar to that during isometric contractions or relaxations. L units were never active during either plantar or maintained flexion, but discharged during lengthening contraction in a given range of rotation velocities; the velocity of lengthening consistently influenced the firing frequency of these units. Such dependence on velocity was not observed in S + L units. 4. A correlation was found between the amplitude of the action potential and the threshold torque of recruitment among all the units. In addition, the amplitudes of both the action potential and the threshold torque were higher in the case of L units than in the case of S and S + L units. Most L units could be voluntarily recruited only in the case of ballistic isometric or isotonic contraction. 5. Occasionally, L units were directly activated by electrical stimulation of motor fibres and their conduction velocity was in the higher range for alpha-axons. In contrast, nerve stimulation could induce a reflex activation of S and S + L units.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., Binder M. D., Ruenzel P., Henneman E. Recruitment order of motoneurons in stretch reflexes is highly correlated with their axonal conduction velocity. J Neurophysiol. 1984 Sep;52(3):410–420. doi: 10.1152/jn.1984.52.3.410. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F. Normal sensory conduction in the nerves of the leg in man. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):404–414. doi: 10.1136/jnnp.34.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F., Woods J. J., Johansson R., Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983 Dec;50(6):1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Broman H., De Luca C. J., Mambrito B. Motor unit recruitment and firing rates interaction in the control of human muscles. Brain Res. 1985 Jul 1;337(2):311–319. doi: 10.1016/0006-8993(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdingen H. J., Freund H. J. The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflugers Arch. 1976 Mar 11;362(1):61–67. doi: 10.1007/BF00588682. [DOI] [PubMed] [Google Scholar]
- Calancie B., Bawa P. Voluntary and reflexive recruitment of flexor carpi radialis motor units in humans. J Neurophysiol. 1985 May;53(5):1194–1200. doi: 10.1152/jn.1985.53.5.1194. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J. Control properties of motor units. J Exp Biol. 1985 Mar;115:125–136. doi: 10.1242/jeb.115.1.125. [DOI] [PubMed] [Google Scholar]
- De Luca C. J., Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol. 1987 Sep;58(3):525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]
- Denier van der Gon J. J., ter Haar Romeny B. M., van Zuylen E. J. Behaviour of motor units of human arm muscles: differences between slow isometric contraction and relaxation. J Physiol. 1985 Feb;359:107–118. doi: 10.1113/jphysiol.1985.sp015577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E. Size principle of motoneuron recruitment and the calibration of muscle force and speed in man. Adv Neurol. 1983;39:227–251. [PubMed] [Google Scholar]
- Ducati A., Parmiggiani F., Schieppati M. Simulation of post-tetanic potentiation and fatigue in muscle using a visco-elastic model. Biol Cybern. 1982;44(2):129–133. doi: 10.1007/BF00317972. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Rothwell J. C. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987 Oct;110(Pt 5):1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Garnett R. A., O'Donovan M. J., Stephens J. A., Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979 Feb;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel D., Arsenault A. B., Lambert J. Soleus-gastrocnemius synergies in controlled contractions produced around the ankle and knee joints: an EMG study. Electromyogr Clin Neurophysiol. 1987 Oct-Nov;27(6-7):405–413. [PubMed] [Google Scholar]
- Grimby L. Firing properties of single human motor units during locomotion. J Physiol. 1984 Jan;346:195–202. doi: 10.1113/jphysiol.1984.sp015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L., Hannerz J. Firing rate and recruitment order of toe extensor motor units in different modes of voluntary conraction. J Physiol. 1977 Jan;264(3):865–879. doi: 10.1113/jphysiol.1977.sp011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- Hannerz J. Discharge properties of motor units in relation to recruitment order in voluntary contraction. Acta Physiol Scand. 1974 Jul;91(3):374–385. doi: 10.1111/j.1748-1716.1974.tb05692.x. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Loeb G. E., Sugano N., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. III. Functional segregation in sartorius. J Neurophysiol. 1987 Feb;57(2):554–562. doi: 10.1152/jn.1987.57.2.554. [DOI] [PubMed] [Google Scholar]
- Ingram D. A., Davis G. R., Swash M. Motor nerve conduction velocity distributions in man: results of a new computer-based collision technique. Electroencephalogr Clin Neurophysiol. 1987 Mar;66(3):235–243. doi: 10.1016/0013-4694(87)90072-1. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER R. F. NERVE CONDUCTION STUDIES IN MAN. Neurology. 1963 Dec;13:1021–1030. doi: 10.1212/wnl.13.12.1021. [DOI] [PubMed] [Google Scholar]
- Mavor H., Atcheson J. B. Posterior tibial nerve conduction. Velocity of sensory and motor fibers. Arch Neurol. 1966 Jun;14(6):661–669. doi: 10.1001/archneur.1966.00470120093012. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol. 1973 Apr;230(2):371–390. doi: 10.1113/jphysiol.1973.sp010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A., Schieppati M. Shift of activity from slow to fast muscle during voluntary lengthening contractions of the triceps surae muscles in humans. J Physiol. 1988 Jan;395:363–381. doi: 10.1113/jphysiol.1988.sp016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. B., Carpenter D. O., Henneman E. Orderly recruitment of muscle action potentials. Arch Neurol. 1968 Dec;19(6):591–597. doi: 10.1001/archneur.1968.00480060061008. [DOI] [PubMed] [Google Scholar]
- Person R. S., Kudina L. P. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972 May;32(5):471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Romanò C., Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987 Sep;390:271–284. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M., Nardone A., Musazzi M. Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol. 1986;5(1):59–66. [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28(4):345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Hamm T. M., Vanden Noven S. Partitioning of monosynaptic Ia EPSP connections with motoneurons according to neuromuscular topography: generality and functional implications. Prog Neurobiol. 1988;30(5):437–447. doi: 10.1016/0301-0082(88)90010-x. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K., SEARS T. A., GILLIATT R. W. The range of conduction velocity in normal motor nerve fibers to the small muscles of the hand and foot. J Neurol Neurosurg Psychiatry. 1959 Aug;22:175–181. doi: 10.1136/jnnp.22.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. K., Ross B. H., Calancie B. Human motor-unit recruitment during isometric contractions and repeated dynamic movements. J Neurophysiol. 1987 Jan;57(1):311–324. doi: 10.1152/jn.1987.57.1.311. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Hodgson J. A., Burke R. E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978 Sep;41(5):1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]


