Abstract
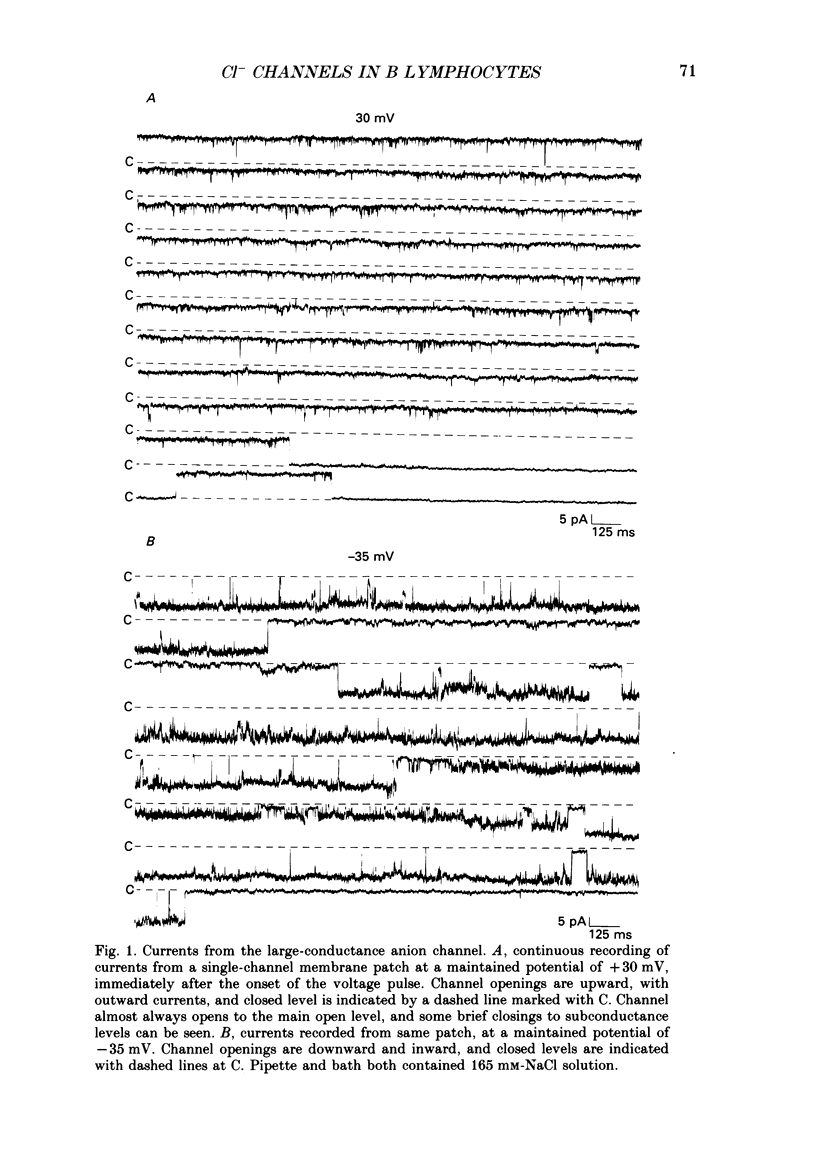
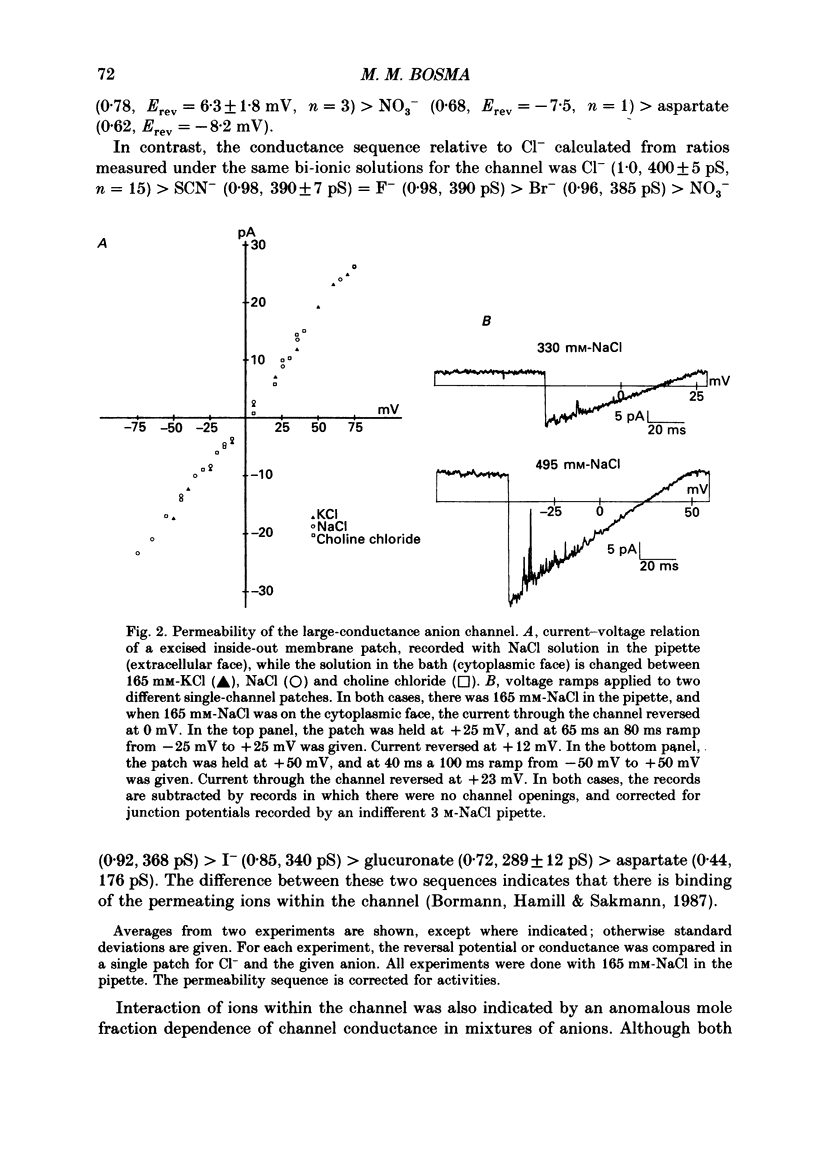
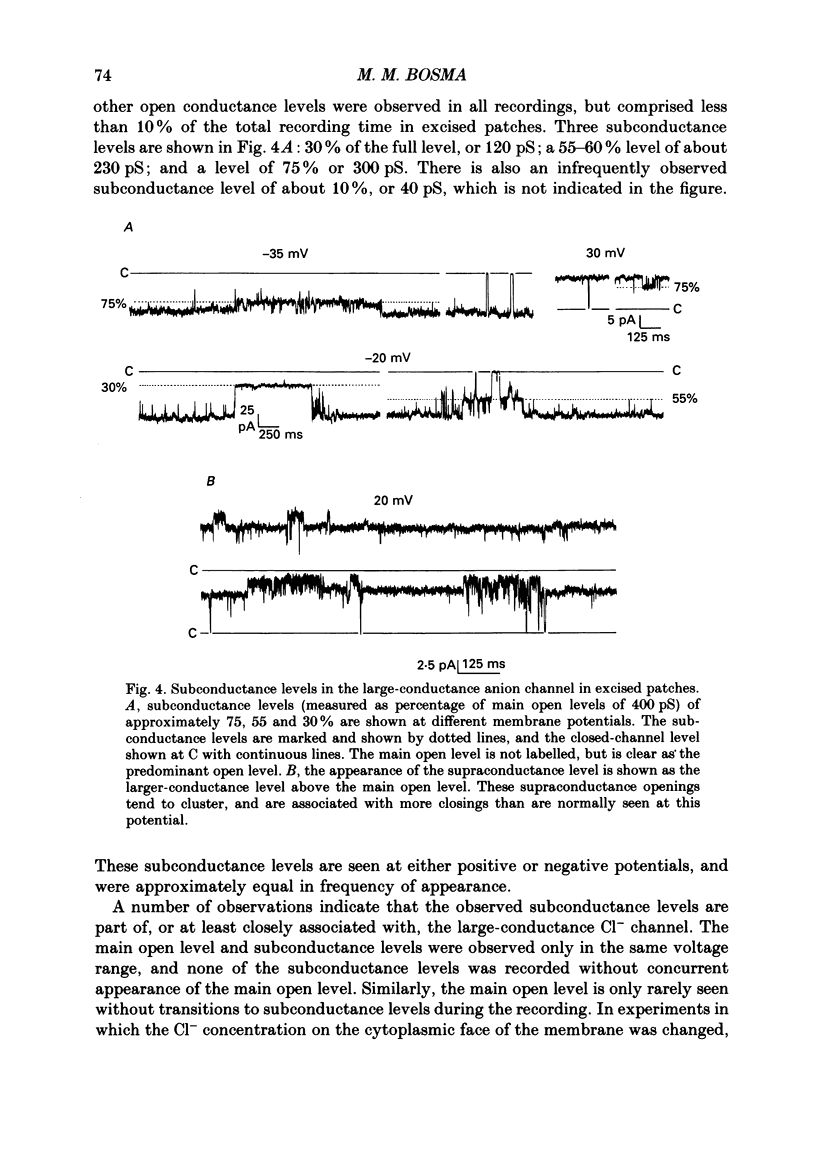
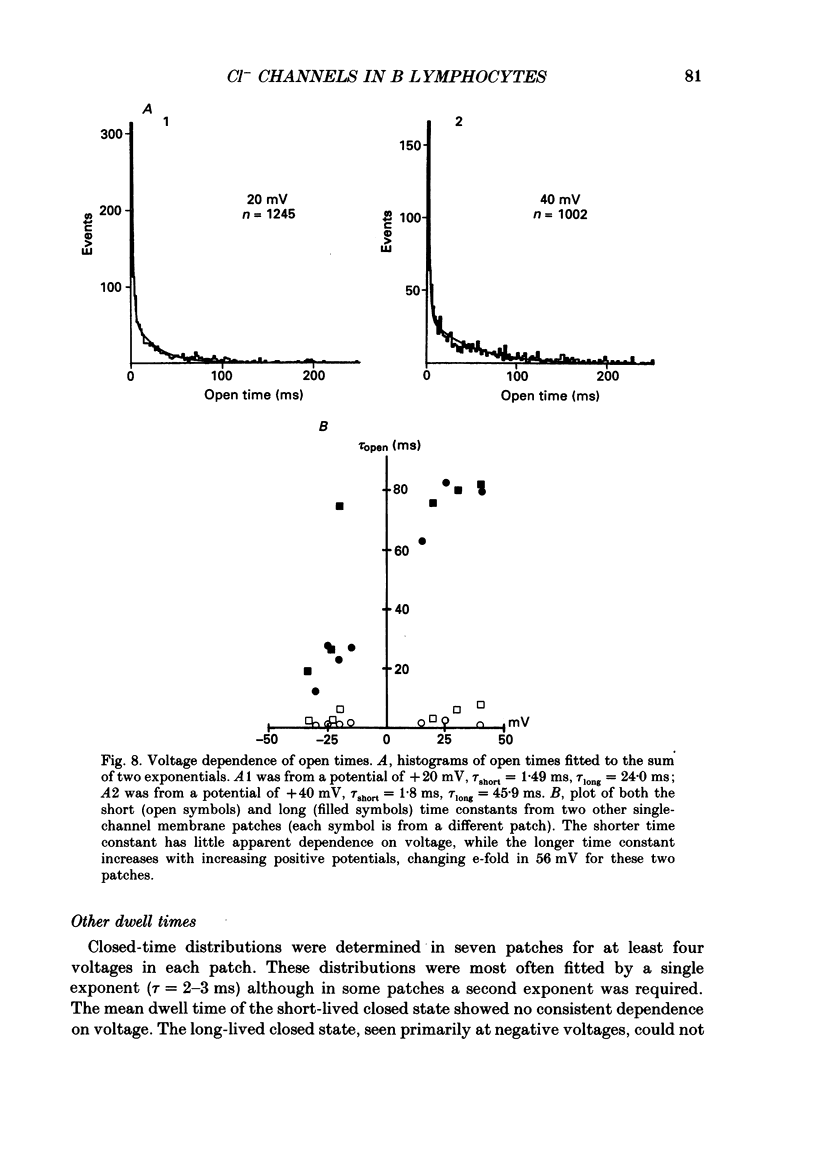
1. Multiple conductance level ion channels were recorded in excised and cell-attached patches from cells of a mouse B lymphocyte hybridoma line. The reversal potential for the single-channel current was unaffected by the species of cation on the cytoplasmic face of the patch, but changed as the Cl- concentration was altered, indicating that the channel is anion selective. 2. The permeability sequence determined from reversal potentials was F- greater than I- greater than SCN- greater than Br- greater than Cl- greater than glucuronate greater than NO3- greater than aspartate. This was different from the conductance sequence (Cl- greater than SCN- = F- greater than Br- greater than NO3- greater than I- greater than glucuronate greater than aspartate), indicating interaction of ions within the pore of the channel. Consistent with this was the observation of anomalous mole fraction dependence with a mixed solution of thiocyanate and chloride. 3. In addition to the main open level (about 400 pS; excised patch, symmetrical 165 mM-Cl-), three subconductance levels and one supraconductance level were observed. These were concluded to be integral components of the same channel based on coincidence of appearance and identical permeabilities. 4. The channel is voltage dependent, with open probability in excised patches increasing with more positive potentials. The channel was reversibly blocked in a voltage-dependent manner by SITS (4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid), a stilbene derivative, on the cytoplasmic face. 5. Several differences were noted between cell-attached and excised-patch recordings. The multiple conductance level channel was less frequently seen in cell-attached patches but could often be induced to appear by prolonged application of positive voltages. This induced channel in attached patches showed an altered voltage dependence which could be partially mimicked in excised patches by including cyclic AMP and ATP in the solution on the cytoplasmic side of the membrane.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Single chloride-selective channels active at resting membrane potentials in cultured rat skeletal muscle. Biophys J. 1985 Jan;47(1):119–123. doi: 10.1016/S0006-3495(85)83884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single voltage-dependent chloride-selective channels of large conductance in cultured rat muscle. Biophys J. 1983 Aug;43(2):237–241. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. H., Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971 Dec;219(2):367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. J Physiol. 1983 Sep;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross N. L., Elinson R. P. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Dev Biol. 1980 Mar;75(1):187–198. doi: 10.1016/0012-1606(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Henkart M. Potassium current in clonal cytotoxic T lymphocytes from the mouse. J Physiol. 1984 Jun;351:645–656. doi: 10.1113/jphysiol.1984.sp015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Saxton R. E. Variation of calcium current during the cell growth cycle in mouse hybridoma lines secreting immunoglobulins. J Physiol. 1984 Oct;355:313–321. doi: 10.1113/jphysiol.1984.sp015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geletyuk V. I., Kazachenko V. N. Single Cl- channels in molluscan neurones: multiplicity of the conductance states. J Membr Biol. 1985;86(1):9–15. doi: 10.1007/BF01871605. [DOI] [PubMed] [Google Scholar]
- Gray P. T., Bevan S., Ritchie J. M. High conductance anion-selective channels in rat cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):395–409. doi: 10.1098/rspb.1984.0041. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Krasne S., Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+ mixtures. J Gen Physiol. 1977 Sep;70(3):269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Mechanism of anion permeation through the muscle fibre membrane of an elasmobranch fish, Taeniura lymma. J Physiol. 1974 Apr;238(1):109–127. doi: 10.1113/jphysiol.1974.sp010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanrahan J. W., Alles W. P., Lewis S. A. Single anion-selective channels in basolateral membrane of a mammalian tight epithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7791–7795. doi: 10.1073/pnas.82.22.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Brown C. D., Murer H. Identification of a voltage-dependent anion channel in the apical membrane of a Cl(-)-secretory epithelium (MDCK). Pflugers Arch. 1985 Mar;403(3):262–265. doi: 10.1007/BF00583597. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Schwarze W. Properties of a cation channel of large unit conductance in lymphocytes, macrophages and cultured muscle cells. Biophys J. 1984 Jan;45(1):136–138. doi: 10.1016/S0006-3495(84)84139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouse M. E., Schneider G. T., Gage P. W. A large anion-selective channel has seven conductance levels. Nature. 1986 Jan 2;319(6048):58–60. doi: 10.1038/319058a0. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Miller C., White M. M. Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1984 May;81(9):2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Schneider G. T., Cook D. I., Gage P. W., Young J. A. Voltage sensitive, high-conductance chloride channels in the luminal membrane of cultured pulmonary alveolar (type II) cells. Pflugers Arch. 1985 Aug;404(4):354–357. doi: 10.1007/BF00585348. [DOI] [PubMed] [Google Scholar]
- Schwarze W., Kolb H. A. Voltage-dependent kinetics of an anionic channel of large unit conductance in macrophages and myotube membranes. Pflugers Arch. 1984 Nov;402(3):281–291. doi: 10.1007/BF00585511. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion interaction at the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1971 Jan;212(2):337–351. doi: 10.1113/jphysiol.1971.sp009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll K. H., Leibowitz M. D., Neumcke B., Hille B. A high-conductance anion channel in adult amphibian skeletal muscle. Pflugers Arch. 1987 Dec;410(6):632–640. doi: 10.1007/BF00581324. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Suzuki N. Blockage of chloride channels by HEPES buffer. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):93–100. doi: 10.1098/rspb.1987.0011. [DOI] [PubMed] [Google Scholar]


