Abstract
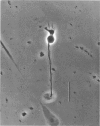
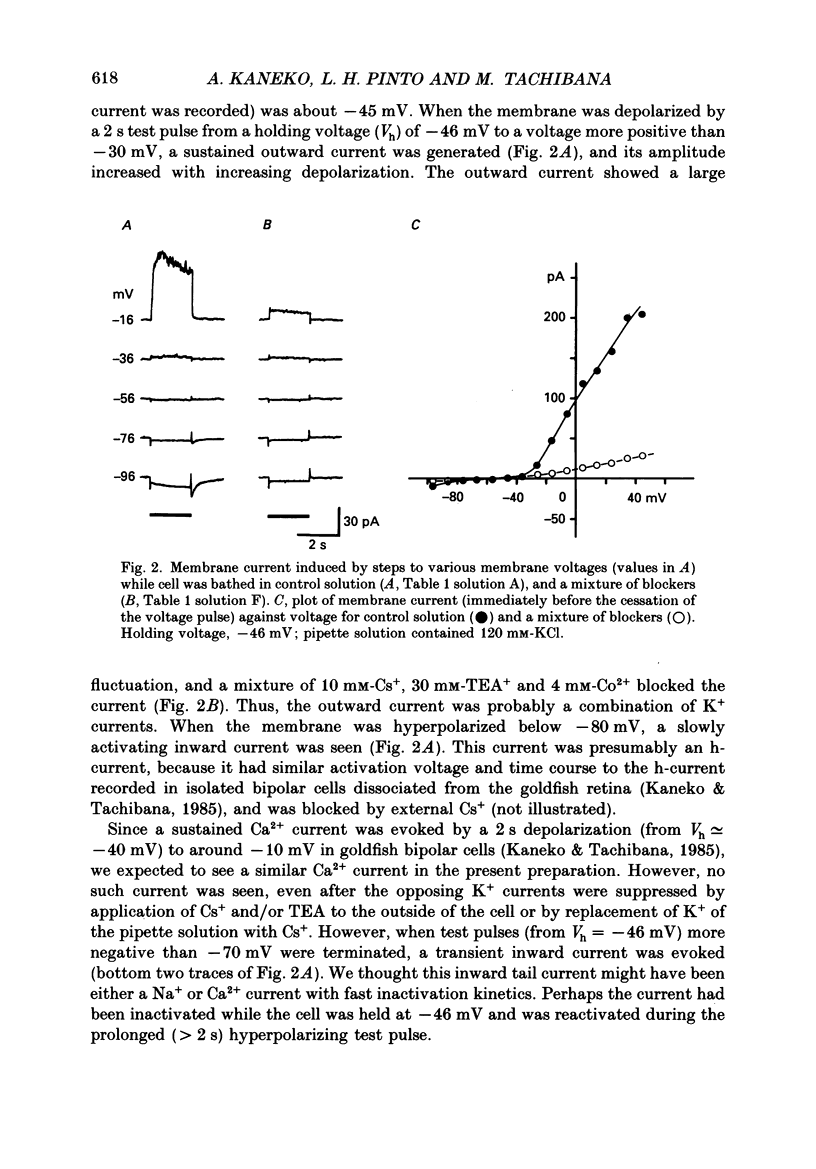
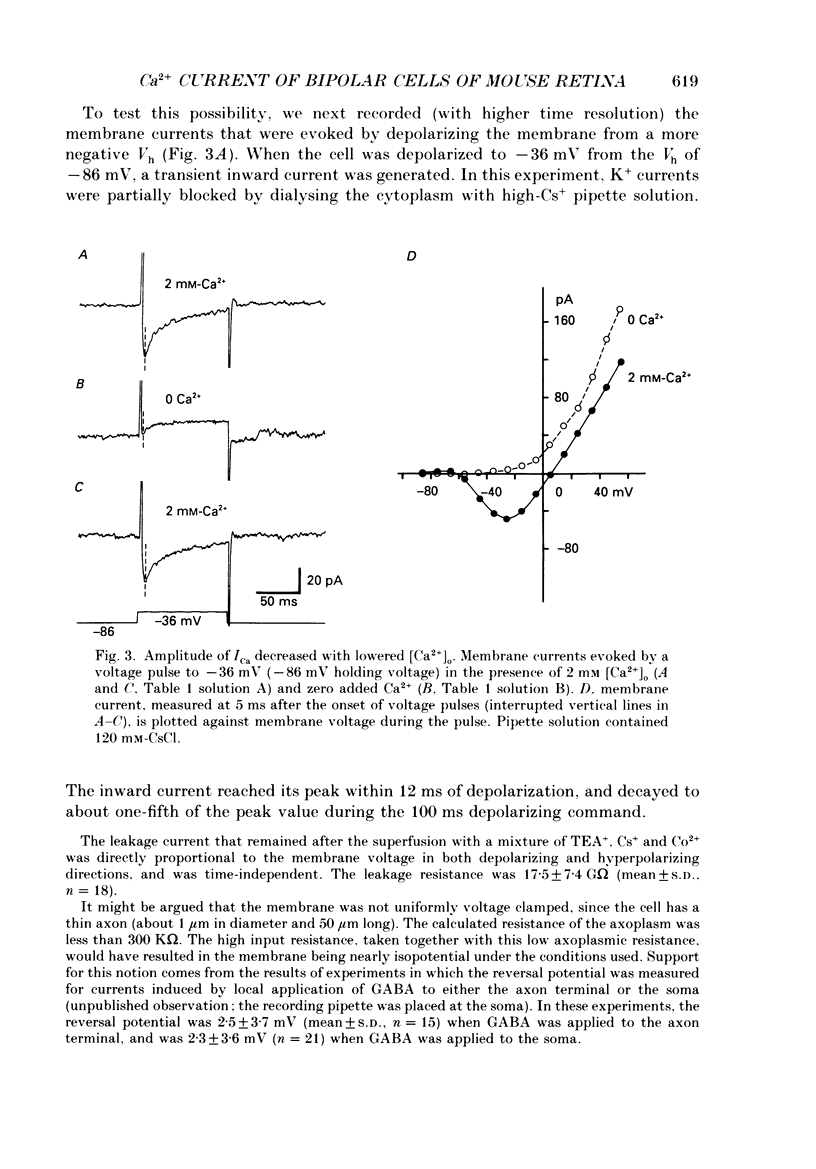
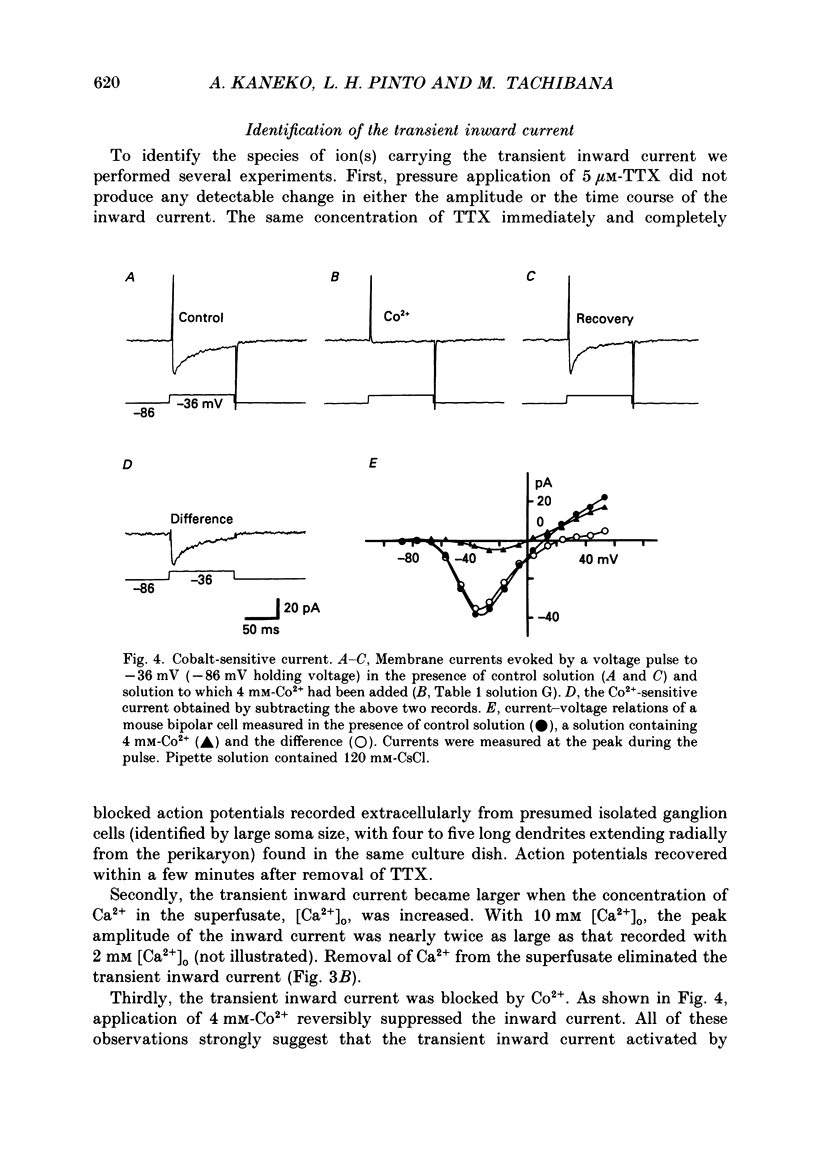
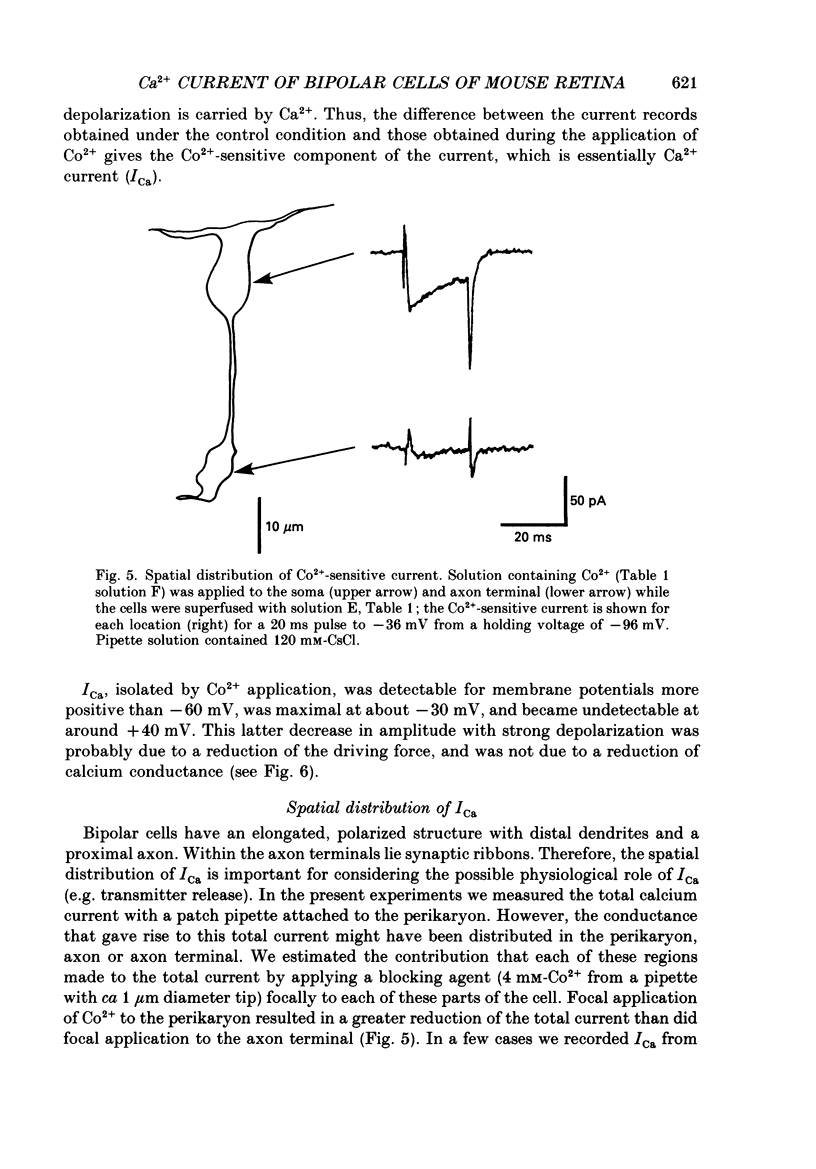
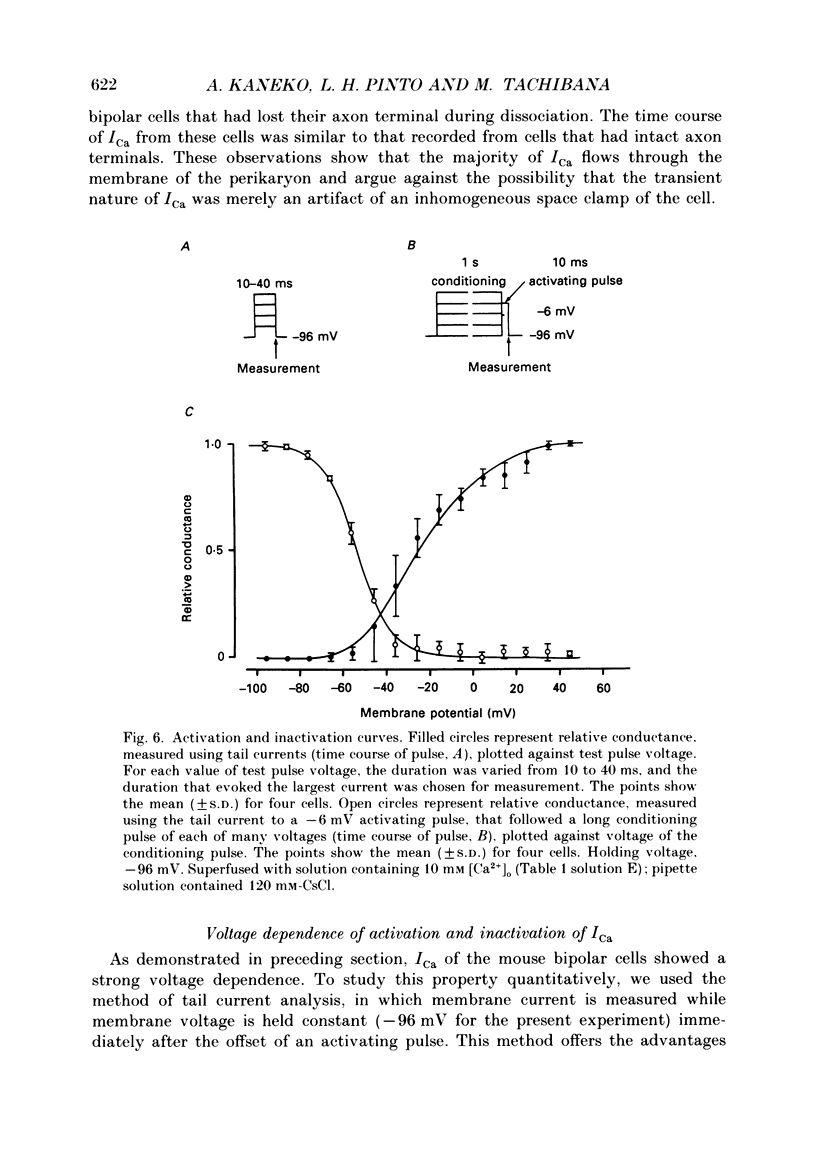
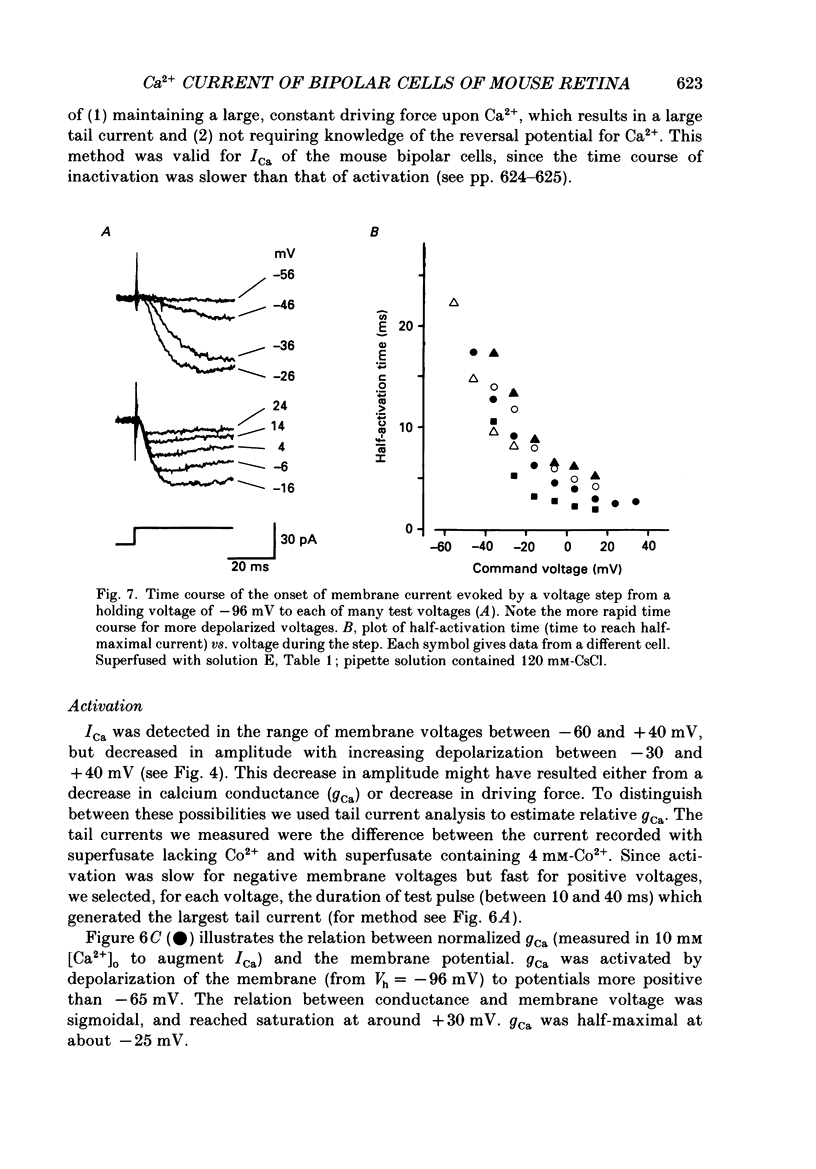
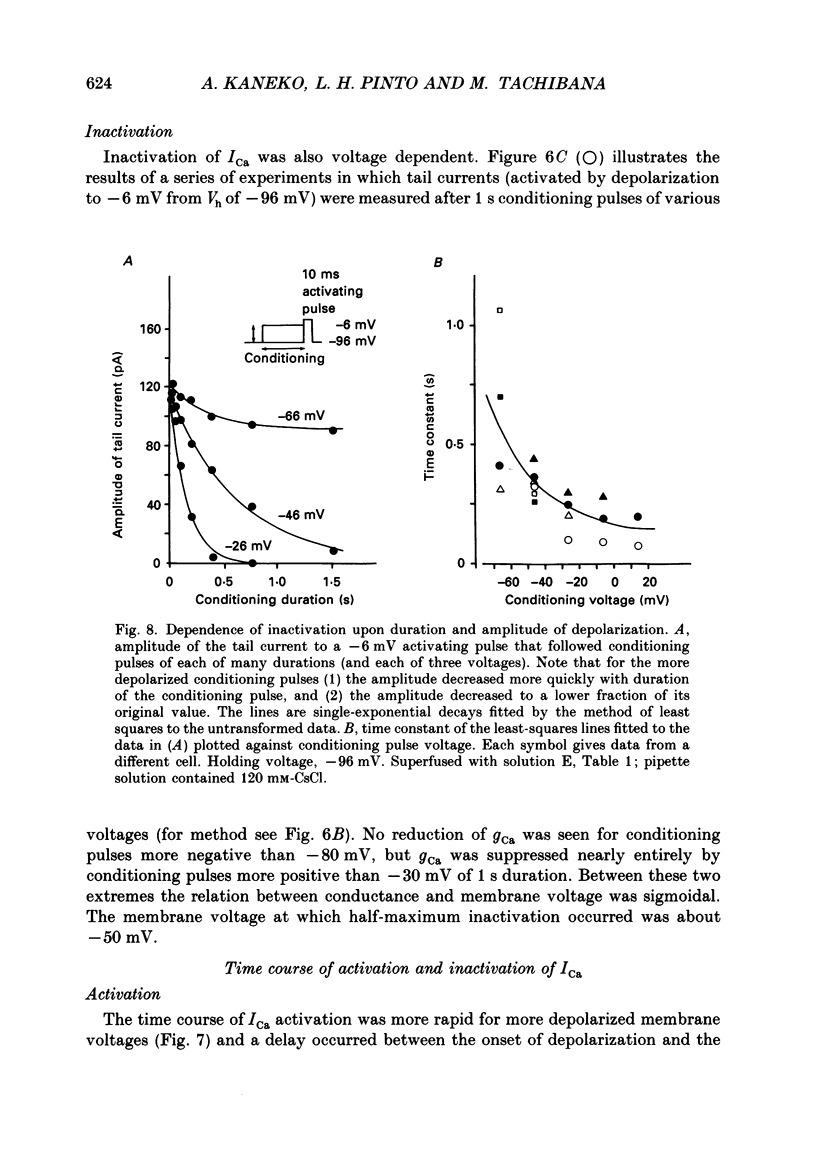
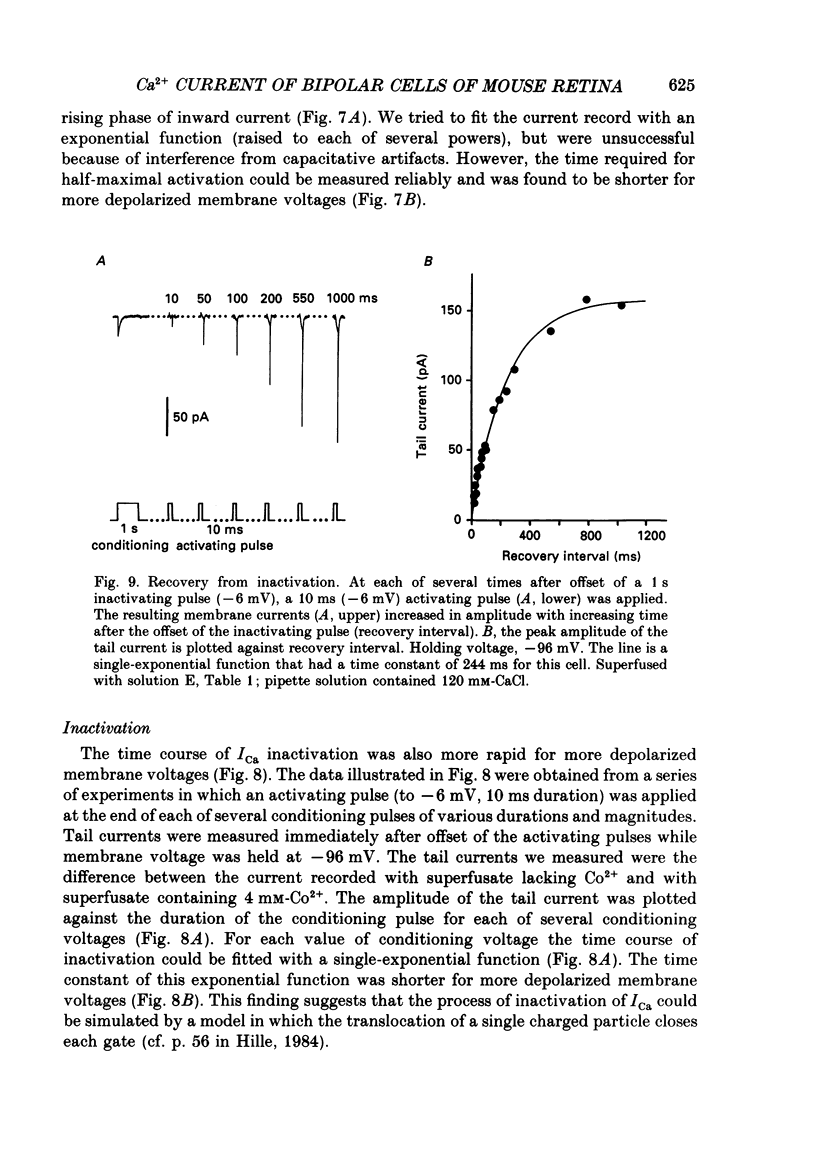
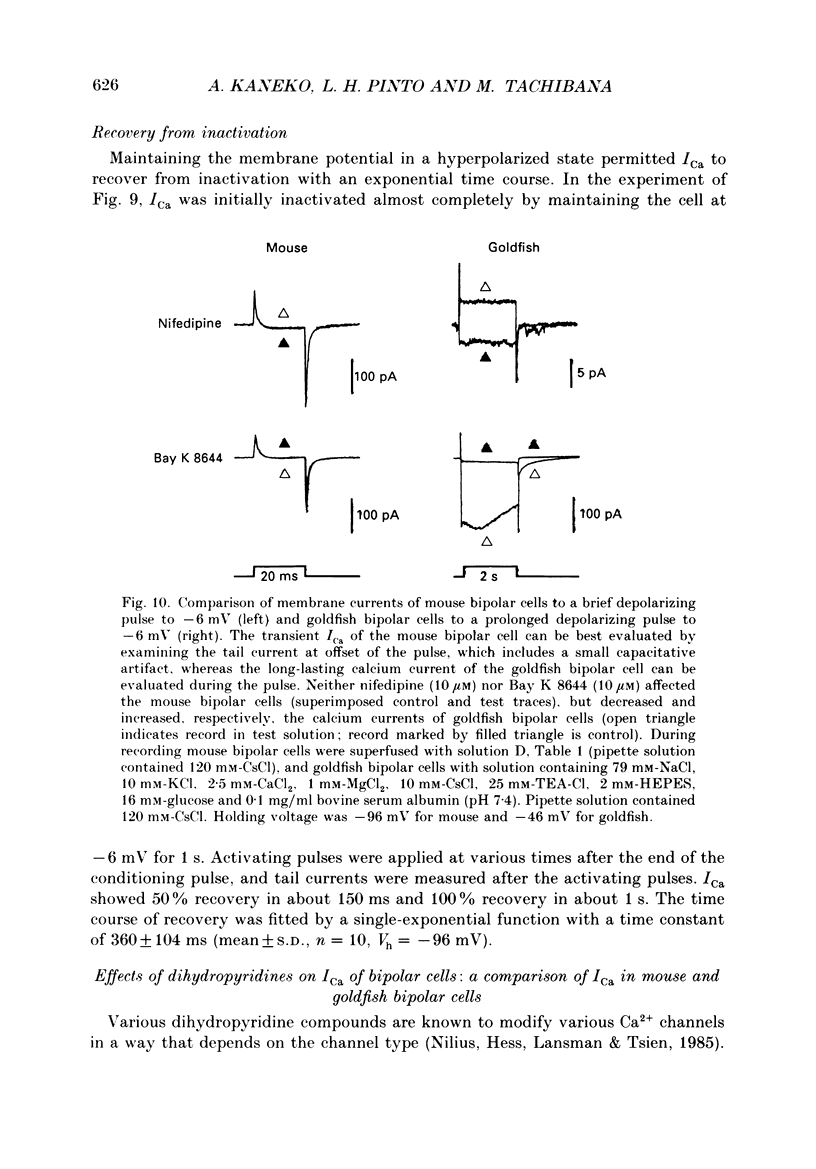
1. Isolated bipolar cells were obtained by enzymic (papain) dissociation of the adult mouse retina. The membrane voltage was clamped and the membrane currents were measured by the whole-cell version of the patch-clamp technique. Isolated bipolar cells and horizontal cells of the goldfish retina were also studied for comparison. 2. Hyperpolarization from the holding voltage, Vh, of -46 mV evoked a slowly activating, Cs+-sensitive, inward current (probably an h-current), and depolarization evoked a TEA- and Cs+-sensitive outward current (probably a combination of K+ currents). 3. Depolarization from a more negative Vh (e.g. -96 mV) evoked a transient inward current that had maximal amplitude between -40 and -20 mV. This current was identified as a Ca2+ current (ICa): its amplitude was increased with elevated [Ca2+]o and was decreased with reduced [Ca2+]o, and it was blocked by 4 mM-Co2+, but not by 5 microM-TTX. 4. Both the perikaryon and the axon terminal generated ICa with similar properties. 5. The plot of Ca2+ conductance (gCa) against membrane voltage (activation curve) was sigmoidal: in 10 mM [Ca2+]o, gCa increased for membrane voltages more positive than -65 mV, was half-maximal at about -25 mV, and reached saturation at about +30 mV. The plot of inactivation of gCa against membrane voltage was also sigmoidal: with 1 s conditioning depolarization in 10 mM [Ca2+]o, gCa decreased for membrane voltages more positive than -80 mV, was half-maximal at about -50 mV, and was fully suppressed for voltages greater than -30 mV. 6. ICa in the mouse bipolar cells was insensitive to 50 microM-Cd2+, 10 microM-nifedipine and 10 microM-Bay K 8644. In contrast, the calcium currents of bipolar and horizontal cells of the goldfish retina were markedly suppressed by 50 microM-Cd2+ and 10 microM-nifedipine, and were augmented several fold by 10 microM-Bay K 8644. The calcium currents of goldfish bipolar and horizontal cells were sustained, and were activated in a more positive range of potentials than the ICa of mouse bipolar cells. 7. The voltage range at which the ICa of mouse bipolar cells is activated includes the presumed range of membrane potentials spanned during light-evoked responses; thus, this current may participate in synaptic transmission. The transient character of ICa may also help to shape transient responses of ganglion cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkema G. W., Mangini N. J., Pinto L. H., Vanable J. W., Jr Visually evoked eye movements in mouse mutants and inbred strains. A screening report. Invest Ophthalmol Vis Sci. 1984 Jul;25(7):795–800. [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977 Dec 23;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Kaneko A., Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol. 1984 Mar;348:255–270. doi: 10.1113/jphysiol.1984.sp015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Potter D. D., Furshpan E. J. Studies on rat sympathetic neurons developing in cell culture. I. Growth characteristics and electrophysiological properties. Dev Biol. 1978 Dec;67(2):384–403. doi: 10.1016/0012-1606(78)90208-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Pinto L. H. Response properties of horizontal cells in the isolated retina of wild-type and pearl mutant mice. J Neurosci. 1986 Apr;6(4):1122–1128. doi: 10.1523/JNEUROSCI.06-04-01122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Permeability changes induced by L-glutamate in solitary retinal horizontal cells isolated from Carassius auratus. J Physiol. 1985 Jan;358:153–167. doi: 10.1113/jphysiol.1985.sp015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]