Abstract
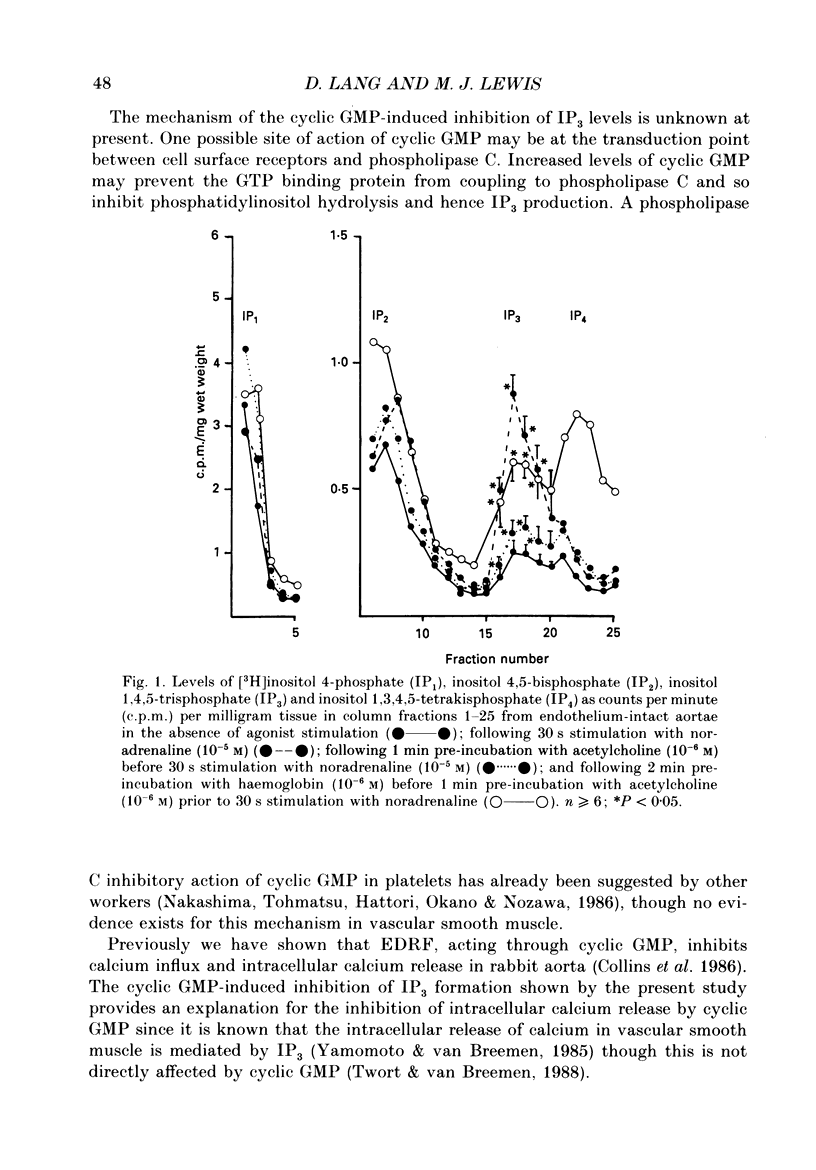
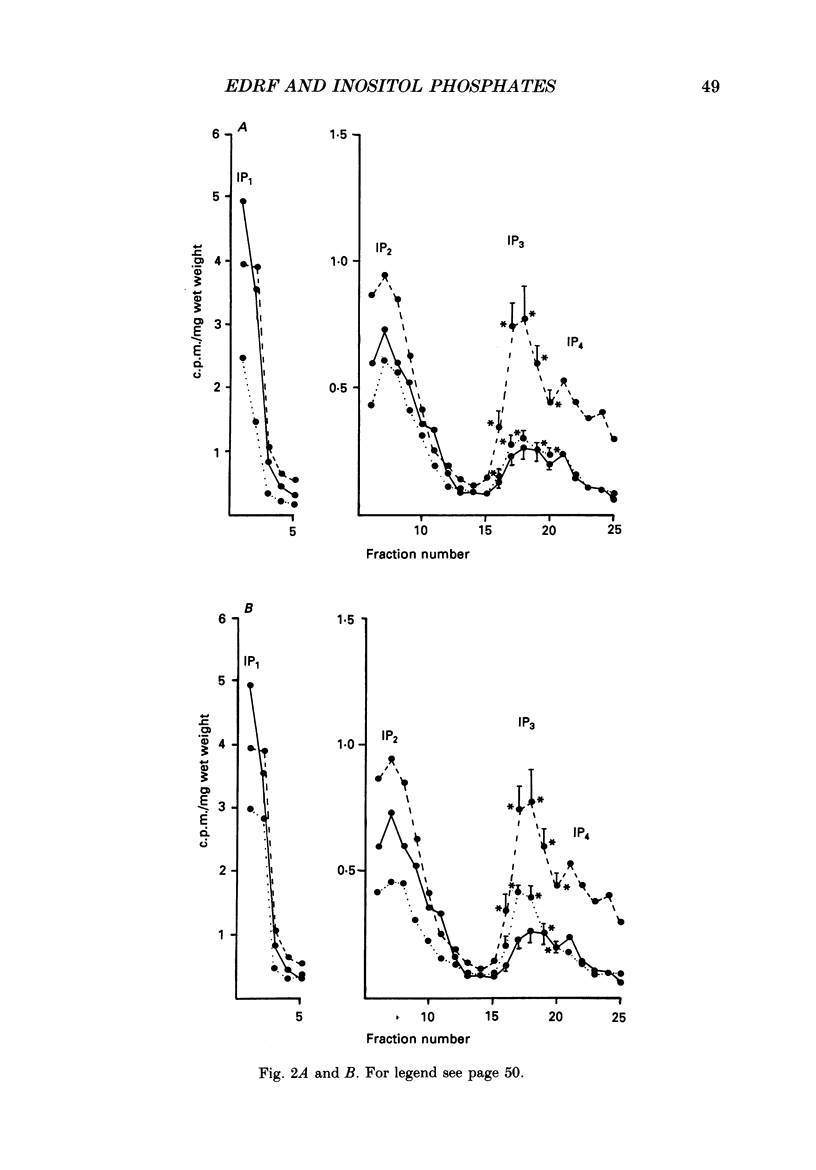
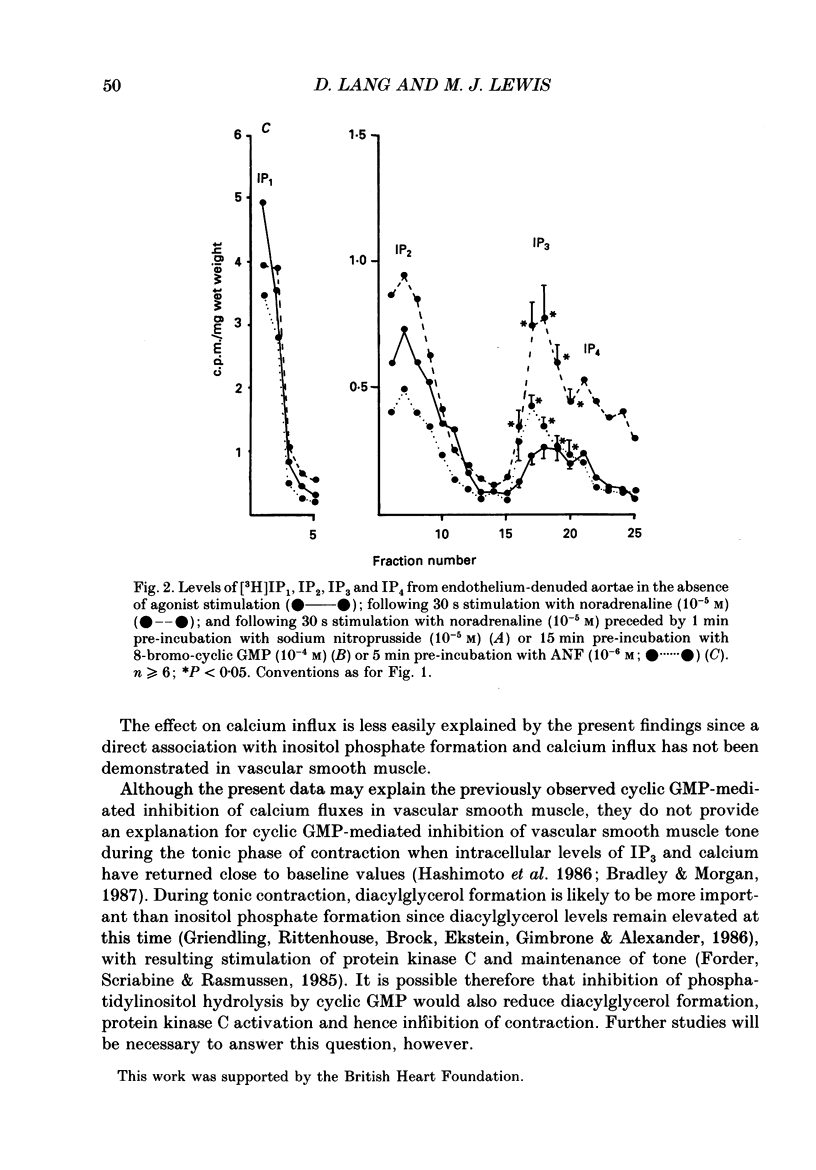
1. The effects of endothelium-derived relaxing factor (EDRF), sodium nitroprusside, 8-bromo-cyclic GMP and atrial natriuretic factor (ANF) on inositol trisphosphate (IP3) levels were studied in isolated rabbit aortic preparations stimulated with noradrenaline. 2. In endothelium-containing preparations, acetylcholine, which stimulated EDRF release, inhibited noradrenaline-stimulated IP3 formation. The EDRF inhibitor haemoglobin reversed this effect. 3. In endothelium-denuded preparations, sodium nitroprusside, 8-bromo-cyclic GMP and ANF each similarly inhibited the rise in IP3 levels stimulated by noradrenaline. 4. These findings show that in rabbit aorta, agents which increase cyclic GMP inhibit the noradrenaline-induced rise in IP3 levels and may provide an explanation for the previously reported observations that cyclic GMP inhibits the noradrenaline-stimulated increase in calcium influx and release of intracellular calcium in vascular smooth muscle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradley A. B., Morgan K. G. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol. 1987 Apr;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Griffith T. M., Henderson A. H., Lewis M. J. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986 Dec;381:427–437. doi: 10.1113/jphysiol.1986.sp016336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder J., Scriabine A., Rasmussen H. Plasma membrane calcium flux, protein kinase C activation and smooth muscle contraction. J Pharmacol Exp Ther. 1985 Nov;235(2):267–273. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Henderson A. H. Evidence that cyclic guanosine monophosphate (cGMP) mediates endothelium-dependent relaxation. Eur J Pharmacol. 1985 Jun 7;112(2):195–202. doi: 10.1016/0014-2999(85)90496-0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985 Jun;233(3):679–685. [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Hattori H., Okano Y., Nozawa Y. Inhibitory action of cyclic GMP on secretion, polyphosphoinositide hydrolysis and calcium mobilization in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1099–1104. doi: 10.1016/0006-291x(86)91041-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effect of sodium-potassium pump inhibitors and membrane-depolarizing agents on sodium nitroprusside-induced relaxation and cyclic guanosine monophosphate accumulation in rat aorta. Circ Res. 1985 Jul;57(1):164–170. doi: 10.1161/01.res.57.1.164. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Matsubara T., Nishizuka Y. Inhibitory action of guanosine 3', 5'-monophosphate on thrombin-induced phosphatidylinositol turnover and protein phosphorylation in human platelets. Biochem Biophys Res Commun. 1981 Jul 16;101(1):61–67. doi: 10.1016/s0006-291x(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Twort C. H., van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988 May;62(5):961–964. doi: 10.1161/01.res.62.5.961. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


