Abstract
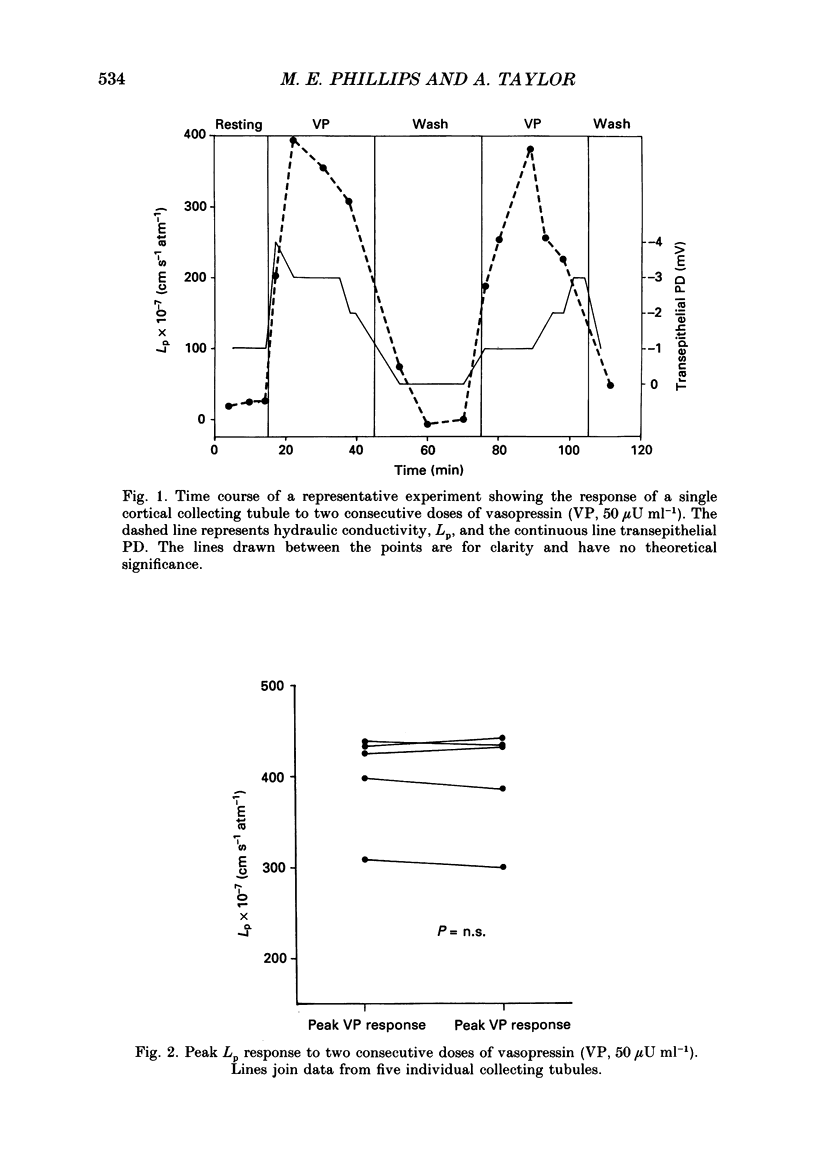
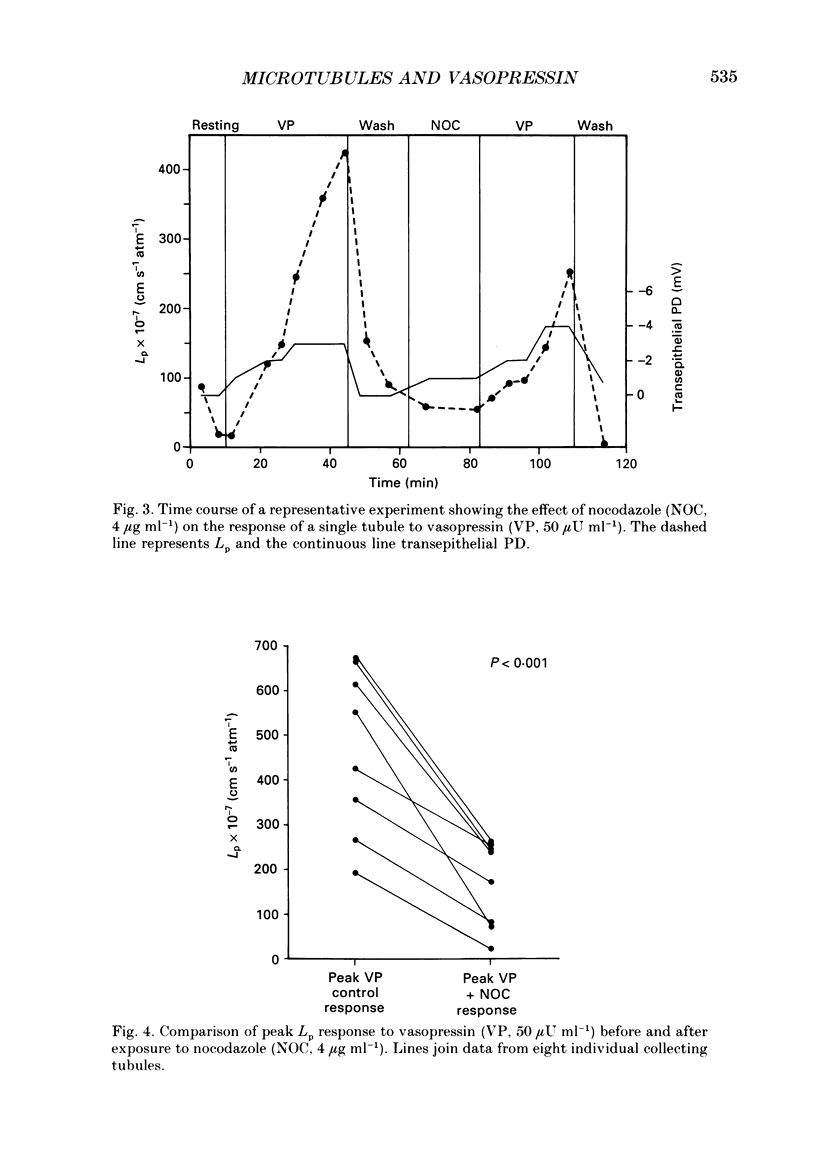
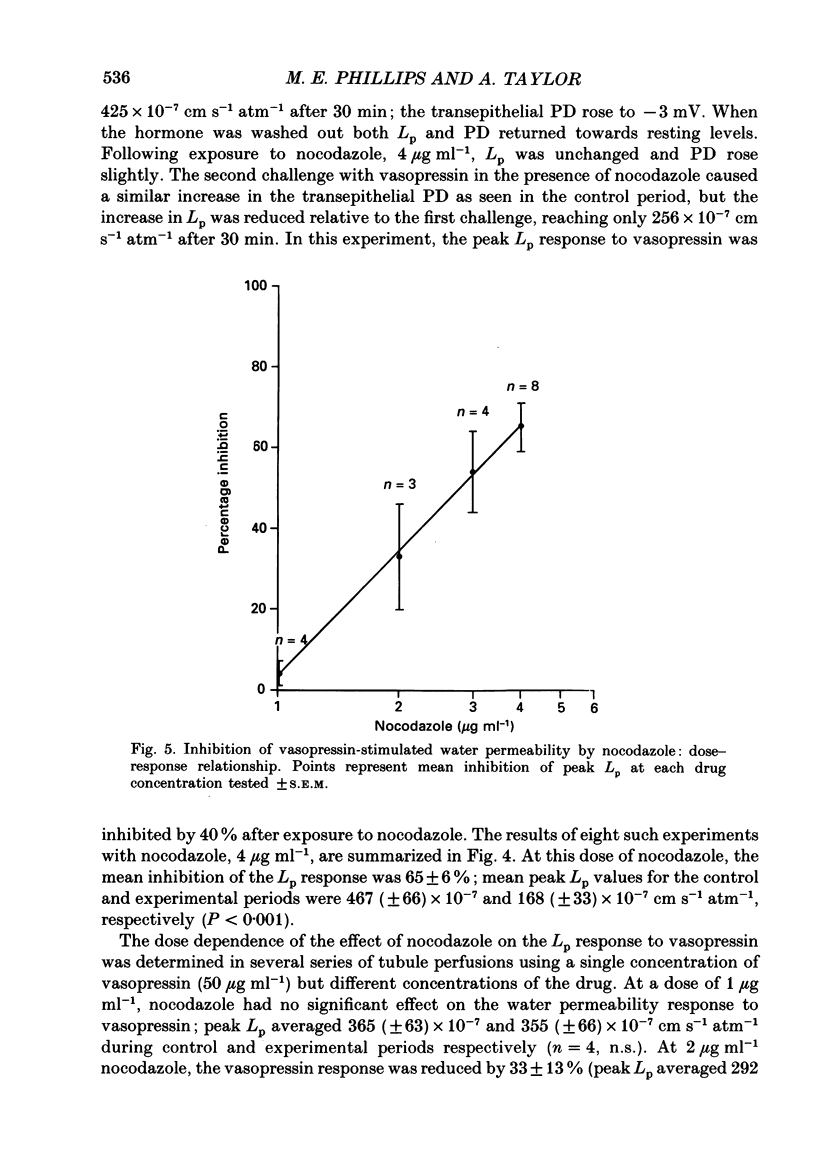
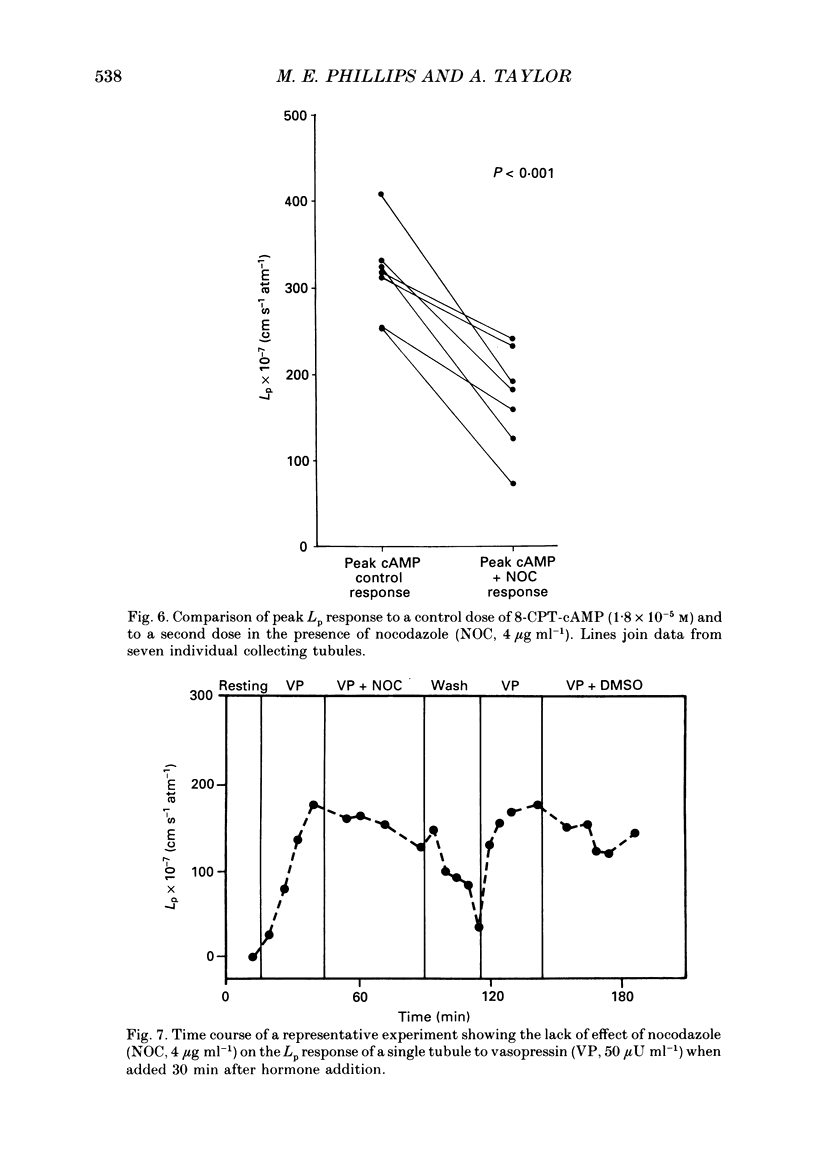
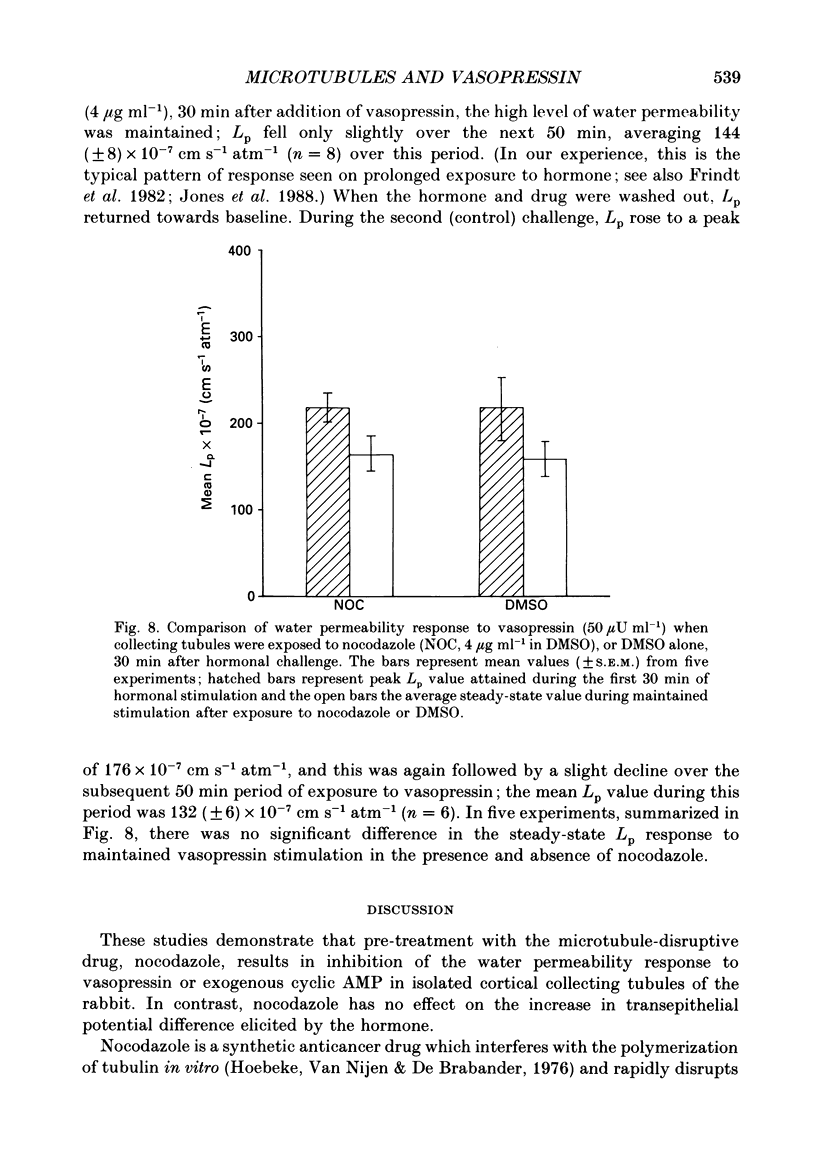
1. The effect of the microtubule-disruptive agent, nocodazole (methyl [5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl] carbamate), on the water permeability response to vasopressin or the synthetic cyclic AMP analogue, 8-parachlorophenylthio-cyclic AMP (8-CPT-cAMP), has been investigated in isolated cortical collecting tubules from rabbit kidneys, perfused in vitro. 2. Pre-treatment with nocodazole, 1-4 micrograms ml-1, had no significant effect on basal water permeability, but inhibited the increase in hydraulic conductivity elicited by vasopressin, 50 microU ml-1, in a dose-dependent manner. Inhibition of the response to the hormone averaged 65 +/- 6% (n = 8, P less than 0.001) at a nocodazole concentration of 4 micrograms ml-1. 3. Nocodazole, 1-4 micrograms ml-1, had no effect on the increase in lumen-negative potential difference (PD) induced by the hormone. 4. Pre-treatment with nocodazole, 4 micrograms ml-1, inhibited the development of the water permeability response to 8-CPT-cAMP, 1.8 x 10(-5) M, by 45 +/- 7% (n = 7, P less than 0.001). 5. When collecting tubules were exposed to nocodazole, 4 micrograms ml-1, after the hydrosmotic response to vasopressin had been fully established, the drug had no inhibitory effect on the maintenance of a high water permeability. 6. The results are consistent with the view that cytoplasmic microtubules play a role in the initiation of the water permeability response to vasopressin in the mammalian cortical collecting tubule at a cellular site beyond the generation of cyclic AMP.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. J., Parsons R. H., Coluccio L. M. Nocodazole inhibition of the vasopressin-induced water permeability increase in toad urinary bladder. Biochim Biophys Acta. 1981 Sep 7;646(3):399–401. doi: 10.1016/0005-2736(81)90308-4. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Halushka P. V. Inhibition of prostaglandin synthesis antagonizes the colchicine-induced reduction of vasopressin-stimulated water flow in the toad urinary bladder. Mol Pharmacol. 1982 Jan;21(1):142–149. [PubMed] [Google Scholar]
- Chevalier J., Bourguet J., Hugon J. S. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 1974;152(2):129–140. doi: 10.1007/BF00224690. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Harris H. W., Jr, Wade J. B. Visualization of endocytosed markers in freeze-fracture studies of toad urinary bladder. J Histochem Cytochem. 1987 Dec;35(12):1405–1414. doi: 10.1177/35.12.3119700. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Effects of colchicine and vinblastine on the cellular action of vasopressin in mammalian kidney. A possible role of microtubules. J Clin Invest. 1974 Aug;54(2):252–262. doi: 10.1172/JCI107760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E., Taylor A. Hydroosmotic response of collecting tubules to ADH or cAMP at reduced peritubular sodium. Am J Physiol. 1982 Nov;243(5):F503–F513. doi: 10.1152/ajprenal.1982.243.5.F503. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Gronowicz G., Masur S. K., Holtzman E. Quantitative analysis of exocytosis and endocytosis in the hydroosmotic response of toad bladder. J Membr Biol. 1980;52(3):221–235. doi: 10.1007/BF01869191. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Barnes L. D., Dousa T. P. Cyclic AMP in action of antidiuretic hormone: effects of exogenous cyclic AMP and its new analogue. Am J Physiol. 1977 Apr;232(4):F368–F376. doi: 10.1152/ajprenal.1977.232.4.F368. [DOI] [PubMed] [Google Scholar]
- Harmanci M. C., Stern P., Kachadorian W. A., Valtin H., DiScala V. A. Vasopressin and collecting duct intramembranous particle clusters: a dose-response relationship. Am J Physiol. 1980 Dec;239(6):F560–F564. doi: 10.1152/ajprenal.1980.239.6.F560. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Fluorescent markers to study membrane retrieval in antidiuretic hormone-treated toad urinary bladder. Am J Physiol. 1986 Aug;251(2 Pt 1):C274–C284. doi: 10.1152/ajpcell.1986.251.2.C274. [DOI] [PubMed] [Google Scholar]
- Hoebeke J., Van Nijen G., De Brabander M. Interaction of oncodazole (R 17934), a new antitumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):319–324. doi: 10.1016/0006-291x(76)90524-6. [DOI] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Lepper K. G., Mailman D. S. Involvement of microtubules and microfilaments in the action of vasopressing in canine renal medulla. J Supramol Struct. 1976;5(4):521(373)–530(382). doi: 10.1002/jss.400050409. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Frindt G., Windhager E. E. Effect of peritubular [Ca] or ionomycin on hydrosmotic response of CCTs to ADH or cAMP. Am J Physiol. 1988 Feb;254(2 Pt 2):F240–F253. doi: 10.1152/ajprenal.1988.254.2.F240. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Ellis S. J., Muller J. Possible roles for microtubules and microfilaments in ADH action on toad urinary bladder. Am J Physiol. 1979 Jan;236(1):F14–F20. doi: 10.1152/ajprenal.1979.236.1.F14. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Wade J. B., DiScala V. A. Vasopressin: induced structural change in toad bladder luminal membrane. Science. 1975 Oct 3;190(4209):67–69. doi: 10.1126/science.809840. [DOI] [PubMed] [Google Scholar]
- Lorenzen M., Taylor A., Windhager E. E. pH effect on osmotic response of collecting tubules to vasopressin and 8-CPT-cAMP. Am J Physiol. 1983 Aug;245(2):F188–F197. doi: 10.1152/ajprenal.1983.245.2.F188. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Holtzman E., Schwartz I. L., Walter R. Correlation between pinocytosis and hydroosmosis induced by neurohypophyseal hormones and mediated by adenosine 3',5'-cyclic monophosphate. J Cell Biol. 1971 Jun;49(3):582–594. doi: 10.1083/jcb.49.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A., DiScala V. A. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol. 1980 Apr;85(1):83–95. doi: 10.1083/jcb.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Lorenzen M. Antidiuretic hormone-dependent membrane capacitance and water permeability in the toad urinary bladder. Am J Physiol. 1983 Feb;244(2):F195–F204. doi: 10.1152/ajprenal.1983.244.2.F195. [DOI] [PubMed] [Google Scholar]
- Parisi M., Pisam M., Mérot J., Chevalier J., Bourguet J. The role of microtubules and microfilaments in the hydrosmotic response to antidiuretic hormone. Biochim Biophys Acta. 1985 Jul 25;817(2):333–342. doi: 10.1016/0005-2736(85)90036-7. [DOI] [PubMed] [Google Scholar]
- Pearl M., Taylor A. Role of the cytoskeleton in the control of transcellular water flow by vasopressin in amphibian urinary bladder. Biol Cell. 1985;55(3):163–172. doi: 10.1111/j.1768-322x.1985.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Reaven E., Maffly R., Taylor A. Evidence for involvement of microtubules in the action of vasopressin in toad urinary bladder. III. Morphological studies on the content and distribution of microtubules in bladder epithelial cells. J Membr Biol. 1978 May 3;40(3):251–267. doi: 10.1007/BF02002971. [DOI] [PubMed] [Google Scholar]
- Strange K., Willingham M. C., Handler J. S., Harris H. W., Jr Apical membrane endocytosis via coated pits is stimulated by removal of antidiuretic hormone from isolated, perfused rabbit cortical collecting tubule. J Membr Biol. 1988 Jul;103(1):17–28. doi: 10.1007/BF01871929. [DOI] [PubMed] [Google Scholar]
- Taylor A., Mamelak M., Golbetz H., Maffly R. Evidence for involvement of microtubules in the action of vasopressin in toad urinary bladder. I. Functional studies on the effects of antimitotic agents on the response to vasopressin. J Membr Biol. 1978 May 3;40(3):213–235. doi: 10.1007/BF02002969. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Lencer W. I., Brown D., Ausiello D. A. Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature. 1988 May 19;333(6170):268–269. doi: 10.1038/333268a0. [DOI] [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- Wilson L., Taylor A. Evidence for involvement of microtubules in the action of vasopressin in toad urinary bladder. II. Colchicine binding properties of toad bladder epithelial cell tubulin. J Membr Biol. 1978 May 3;40(3):237–250. doi: 10.1007/BF02002970. [DOI] [PubMed] [Google Scholar]


