Abstract
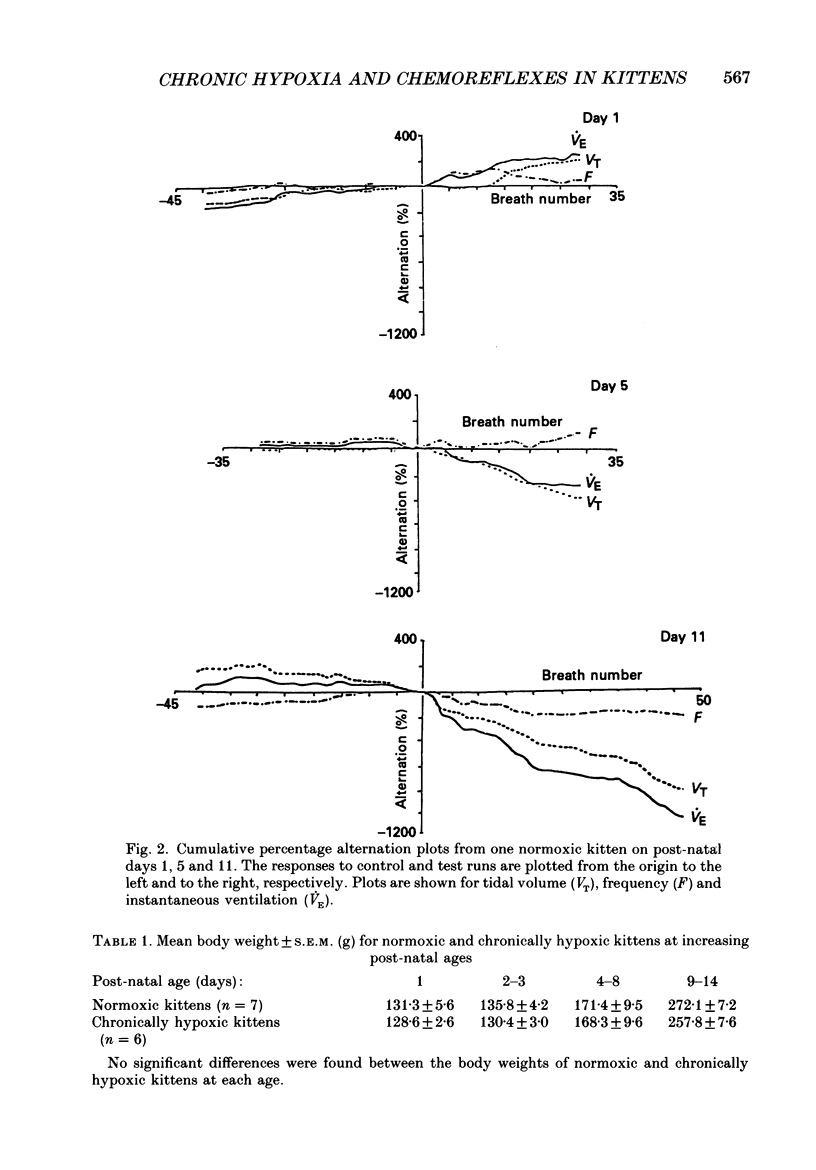
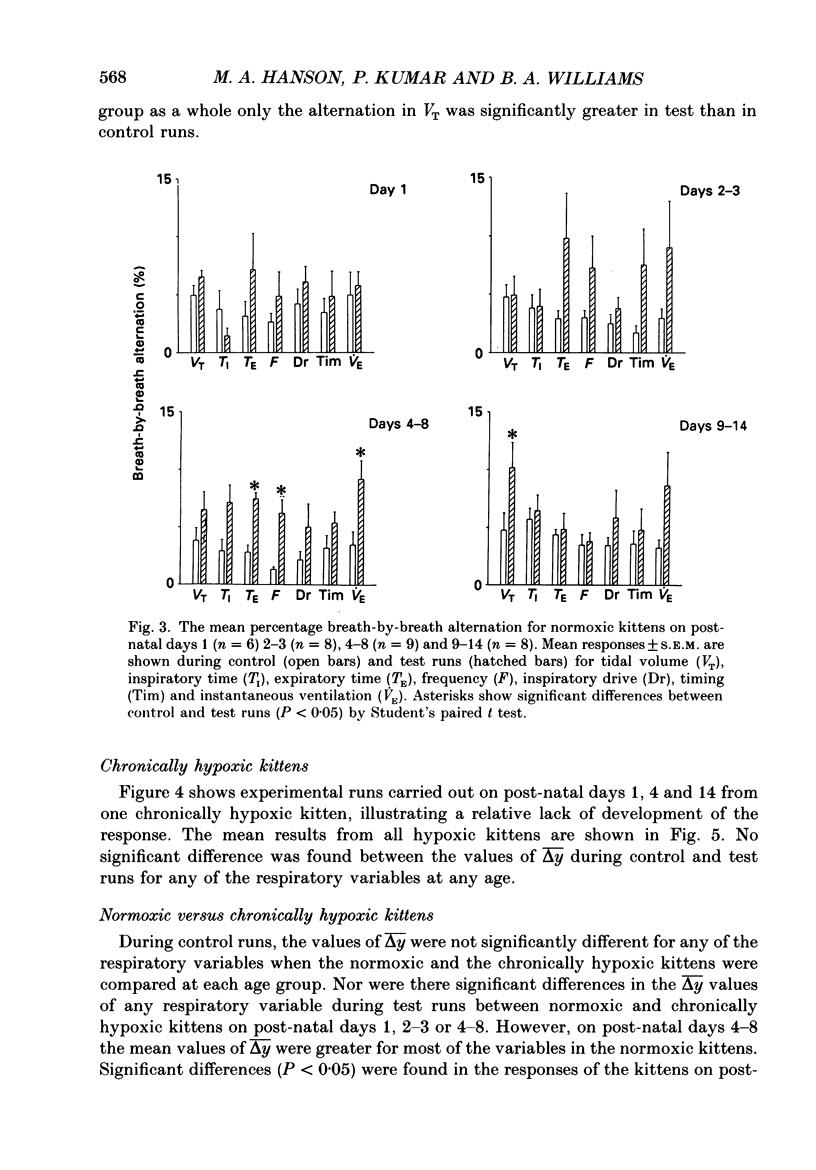
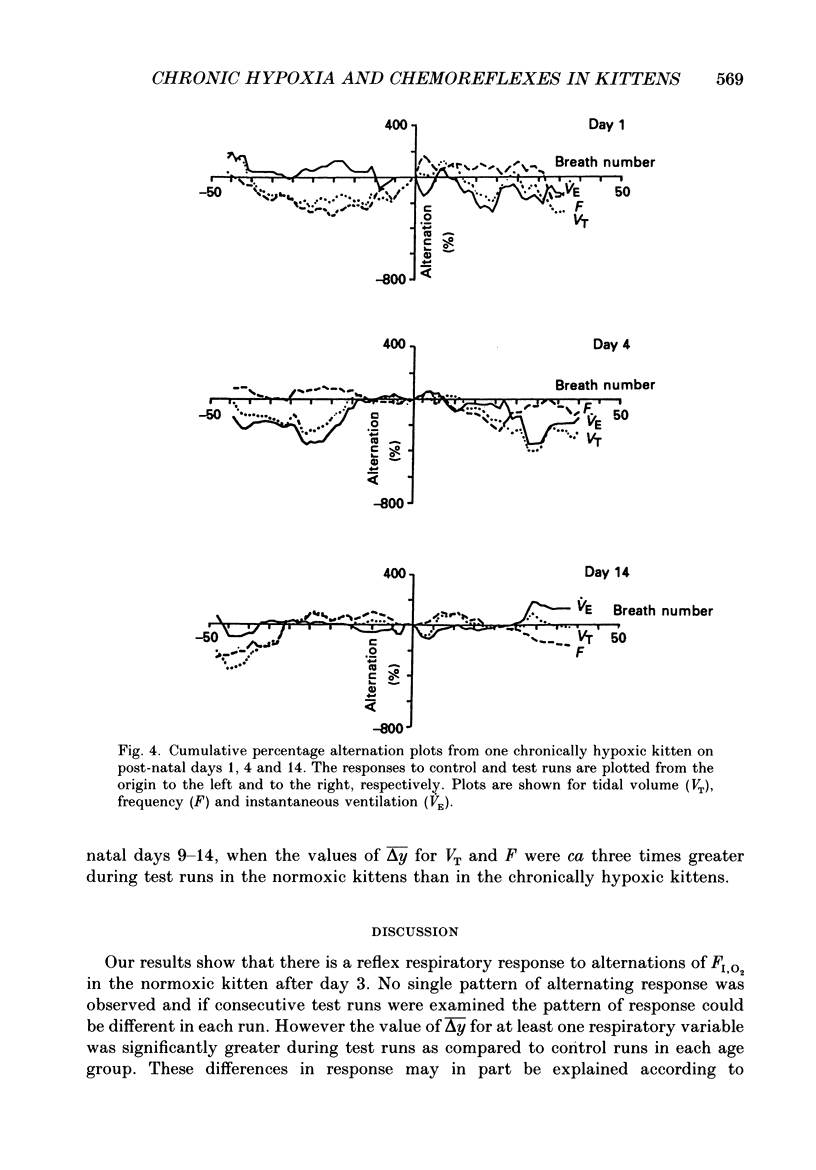
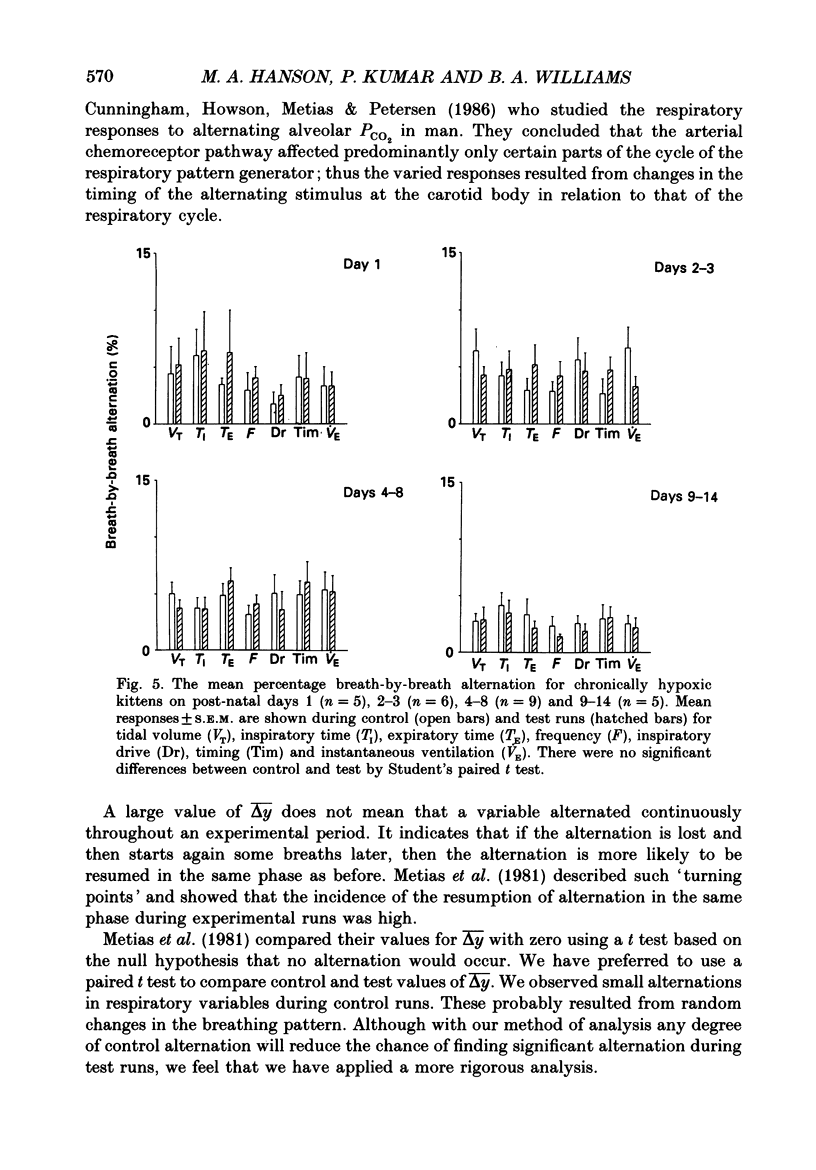
1. We have studied the reflex respiratory responses to two-breath alternations of fractional inspired oxygen (FI,O2) in normoxic (reared in room air) and chronically hypoxic kittens (born into and reared in an FI,O2 of 0.13-0.15) on post-natal days 1, 2-3, 4-8 and 9-14. 2. Respiration was measured non-invasively in the unanaesthetized kittens. Test runs (with alternations of FI,O2 between 0.21 and 0.14) and control runs (with an FI,O2 of 0.21) were carried out during quiet sleep. 3. The degree of alternation in tidal volume, inspiratory time, expiratory time, frequency, drive, timing and instantaneous ventilation components of the respiratory response was compared during control and test runs. 4. There was little response to control runs in either group at any post-natal age. 5. In normoxic kittens we found no significant reflex response in any respiratory variable to test runs before day 4. However significant alternation was found in expiratory time, frequency and ventilation at days 4-8 and in tidal volume at days 9-14. 6. In chronically hypoxic kittens there were no significant differences between control and test runs at any of the ages studied. 7. In the normoxic group increases in the response with post-natal age probably reflect post-natal increases in the sensitivity of the peripheral chemoreceptors. The lack of development in the chronically hypoxic group may indicate abnormal function or delayed maturation of the peripheral chemoreceptor sensitivity to hypoxia. 8. The results suggest that the method can be used to detect developmental and pathological changes in the arterial chemoreflex.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisgard G. E., Ruiz A. V., Grover R. F., Will J. A. Ventilatory acclimatization to 3400 meters altitude in the Hereford calf. Respir Physiol. 1974 Sep;21(3):271–296. doi: 10.1016/0034-5687(74)90059-0. [DOI] [PubMed] [Google Scholar]
- Black A. M., Torrance R. W. Respiratory oscillations in chemoreceptor discharge in the control of breathing. Respir Physiol. 1971 Nov;13(2):221–237. doi: 10.1016/0034-5687(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Dawes G. S., Hanson M. A., McCooke H. B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984 Jun;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C. E., Martin C. B., Jr, Hanson M. A., McCooke H. B. Determinants of the onset of continuous air breathing at birth. Eur J Obstet Gynecol Reprod Biol. 1987 Oct;26(2):183–192. doi: 10.1016/0028-2243(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Cunningham D. J., Howson M. G., Metias E. F., Petersen E. S. Patterns of breathing in response to alternating patterns of alveolar carbon dioxide pressures in man. J Physiol. 1986 Jul;376:31–45. doi: 10.1113/jphysiol.1986.sp016140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Gardner W. N., Johnston B. M., Walker D. W. Breathing in fetal lambs: the effect of brain stem section. J Physiol. 1983 Feb;335:535–553. doi: 10.1113/jphysiol.1983.sp014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S. The central control of fetal breathing and skeletal muscle movements. J Physiol. 1984 Jan;346:1–18. doi: 10.1113/jphysiol.1984.sp015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Johnston B. M. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol. 1987 Jan;382:373–383. doi: 10.1113/jphysiol.1987.sp016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL J. R. The oxygen consumption of new-born and adult mammals. Its dependence on the oxygen tension in the inspired air and on the environmental temperature. J Physiol. 1959 Dec;149:346–373. doi: 10.1113/jphysiol.1959.sp006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. E., McCulloch K., Brouillette R. T. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jun;50(6):1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D., Astic L., Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kumar P., Nye P. C., Torrance R. W. Do oxygen tension variations contribute to the respiratory oscillations of chemoreceptor discharge in the cat? J Physiol. 1988 Jan;395:531–552. doi: 10.1113/jphysiol.1988.sp016933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S., Brody J. S., Motoyama E. K., Velasquez T. M. Regulation of breathing in newborns at high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):673–678. doi: 10.1152/jappl.1978.44.5.673. [DOI] [PubMed] [Google Scholar]
- Marsh R. H., Lyen K. R., McPherson G. A., Pearson S. B., Cunningham D. J. Breath-by-breath effects of imposed alternate-breath oscillations of alveolar CO 2 . Respir Physiol. 1973 Jun;18(1):80–91. doi: 10.1016/0034-5687(73)90023-6. [DOI] [PubMed] [Google Scholar]
- Martin-Body R. L., Johnston B. M. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol. 1988 Jan;71(1):25–32. doi: 10.1016/0034-5687(88)90112-0. [DOI] [PubMed] [Google Scholar]
- McCooke H. B., Hanson M. A. Respiration of conscious kittens in acute hypoxia and effect of almitrine bismesylate. J Appl Physiol (1985) 1985 Jul;59(1):18–23. doi: 10.1152/jappl.1985.59.1.18. [DOI] [PubMed] [Google Scholar]
- Metias E. F., Cunningham D. J., Howson M. G., Petersen E. S., Wolff C. B. Reflex effects on human breathing of breath-by-breath changes of the time profile of alveolar PCO2 during steady hypoxia. Pflugers Arch. 1981 Mar;389(3):243–250. doi: 10.1007/BF00584785. [DOI] [PubMed] [Google Scholar]
- Naeye R. L., Fisher R., Ryser M., Whalen P. Carotid body in the sudden infant death syndrome. Science. 1976 Feb 13;191(4227):567–569. doi: 10.1126/science.1251191. [DOI] [PubMed] [Google Scholar]
- Nye P. C., Hanson M. A., Torrance R. W. The effect on breathing of abruptly stopping carotid body discharge. Respir Physiol. 1981 Dec;46(3):309–326. doi: 10.1016/0034-5687(81)90129-8. [DOI] [PubMed] [Google Scholar]
- Parot S., Bonora M., Gautier H., Marlot D. Developmental changes in ventilation and breathing pattern in unanesthetized kittens. Respir Physiol. 1984 Dec;58(3):253–262. doi: 10.1016/0034-5687(84)90002-1. [DOI] [PubMed] [Google Scholar]
- Shannon D. C., Kelly D. H., O'Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Engl J Med. 1977 Oct 6;297(14):747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Respiratory insensitivity to acute hypoxia persisting after correction of tetralogy of Fallot. J Appl Physiol. 1968 Sep;25(3):221–223. doi: 10.1152/jappl.1968.25.3.221. [DOI] [PubMed] [Google Scholar]
- Ward S. A., Drysdale D. B., Cunningham D. J., Petersen E. S. Inspiratory-expiratory responses to alternate-breath oscillation of PACO2 and PAO2. Respir Physiol. 1979 Apr;36(3):311–325. doi: 10.1016/0034-5687(79)90044-6. [DOI] [PubMed] [Google Scholar]


