Abstract
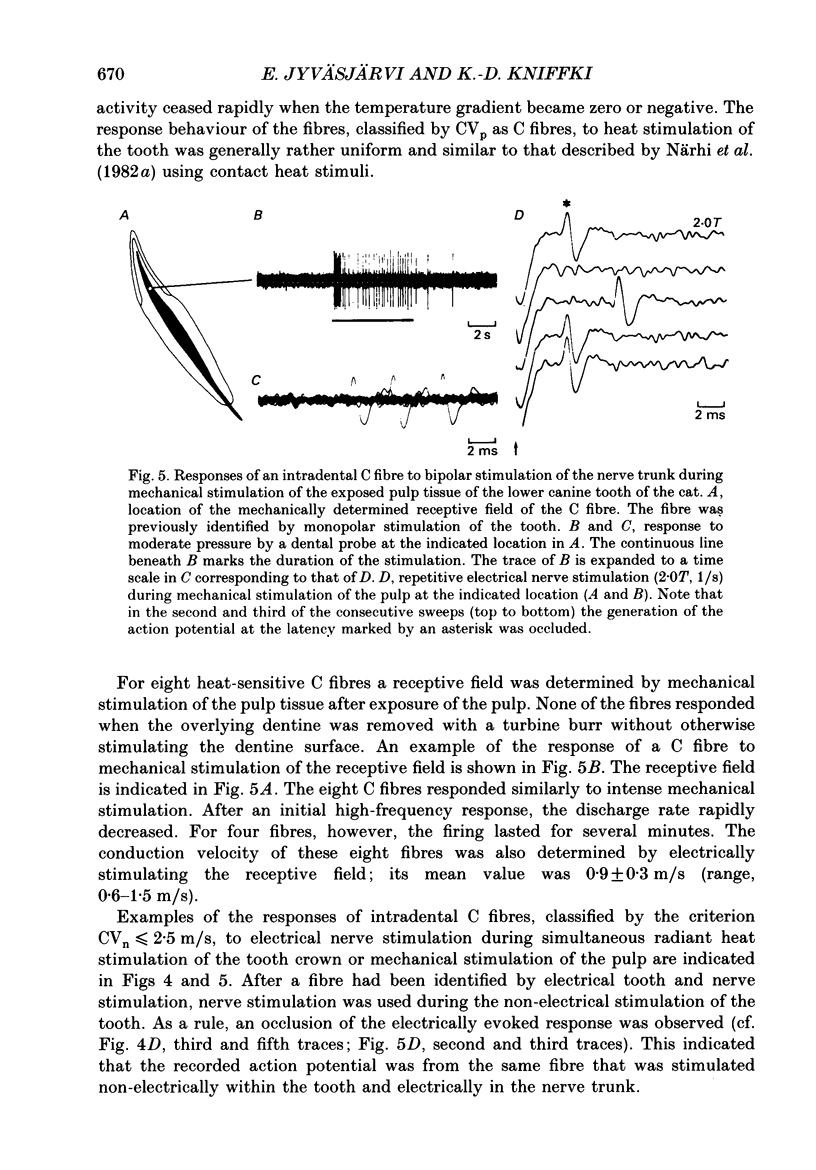
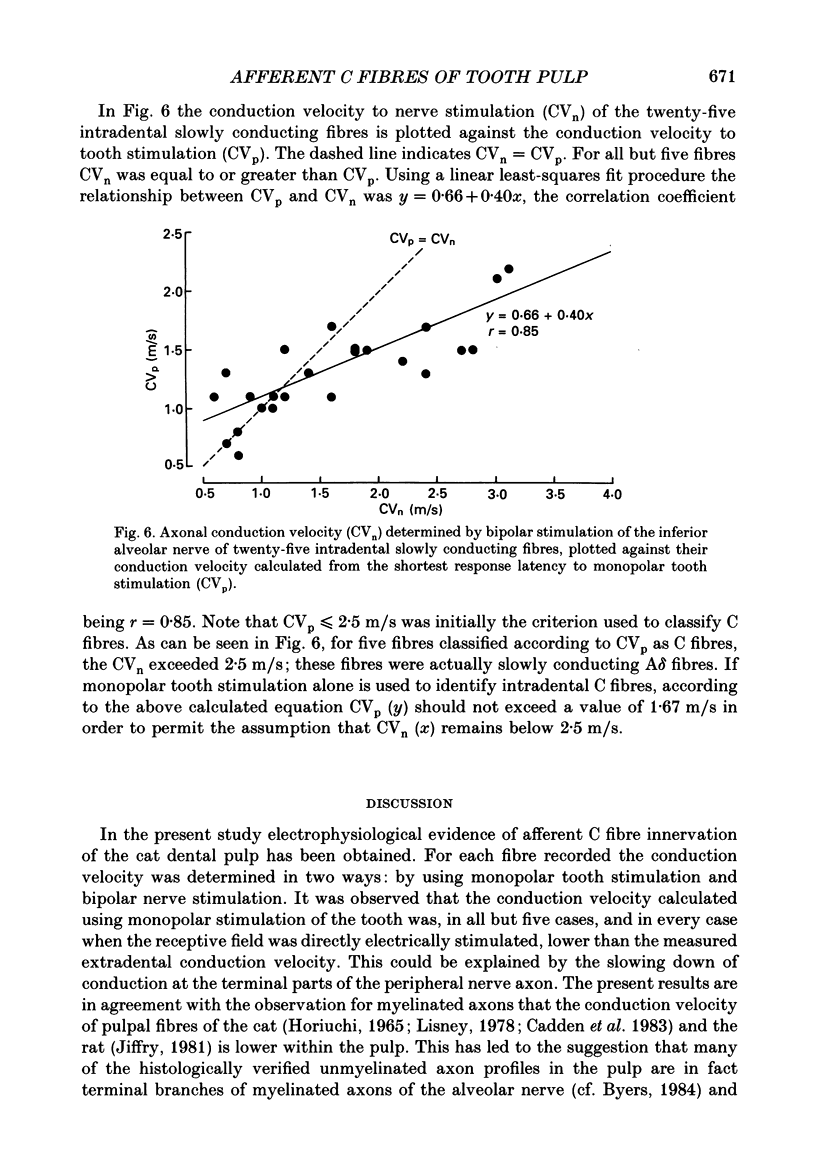
1. The presence of afferent C fibres innervating the lower canine tooth was investigated in Nembutal-anaesthetized cats. 2. Twenty-five single fibres with conduction velocities (CVp) of less than 2.5 m/s, as calculated from the shortest response latency using monopolar electrical stimulation of the tooth, were recorded from the inferior alveolar nerve. In addition, the extradental conduction velocity (CVn) of the fibres was determined by using bipolar electrical stimulation of the trunk of the inferior alveolar nerve. 3. The mean CVp was 1.4 +/- 0.4 m/s (n = 25; range, 0.6-2.4 m/s); the mean CVn was higher, 1.7 +/- 0.9 m/s (n = 25; range, 0.6-4.0 m/s). For 20% of the fibres CVn exceeded 2.5 m/s; these were slowly conducting A delta fibres. For 80% of the fibres, however, the extradental conduction velocity was in the C fibre range. 4. The relationship between CVp (y) and CVn (x) was y = 0.66 + 0.40x, the correlation coefficient being r = 0.85. According to the present results this implies that for a reliable classification of pulpal C fibres (CVn less than or equal to 2.5 m/s) by monopolar tooth stimulation alone, CVp should be less than 1.7 m/s. 5. For twenty-three of the twenty-five fibres, one to three discrete shortenings of the response latency occurred when the intensity of the tooth stimulation was increased. When the nerve trunk itself was stimulated, a constant response latency was measured at all stimulus intensities applied. 6. For twelve fibres tested, the mean rate of electrical stimulation of the tooth, which the response followed with a constant latency, was 4.1 +/- 2.3 Hz (range, 1.5-10.0 Hz). With higher rates of stimulation the response latency increased until the fibres failed to follow each stimulus pulse. 7. Fifteen of the nineteen fibres tested responded to radiant heat stimulation of the tooth they were innervating. The mean temperature threshold was 41.4 +/- 2.7 degrees C (n = 11; range, 37.4 +/- 46.4 degrees C). 8. For eight heat-sensitive pulpal C fibres the receptive field was determined by mechanical stimulation of the exposed pulp tissue. Four C fibres developed a long-lasting on-going discharge after intense mechanical stimulation of the receptive field. 9. The discharge evoked by heat and mechanical stimulation of the tooth occluded the response evoked by simultaneously applied electrical current pulses to the nerve trunk, indicating that the same fibres were activated by both tooth and nerve stimulation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

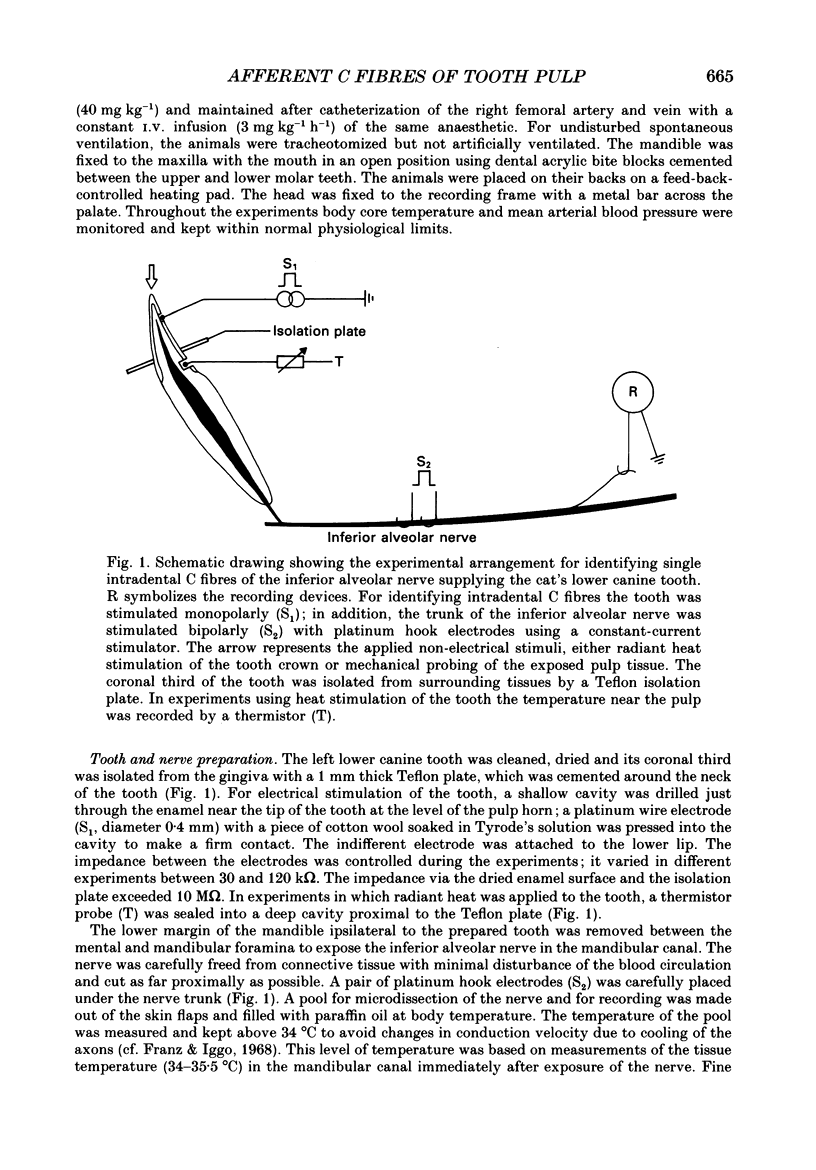

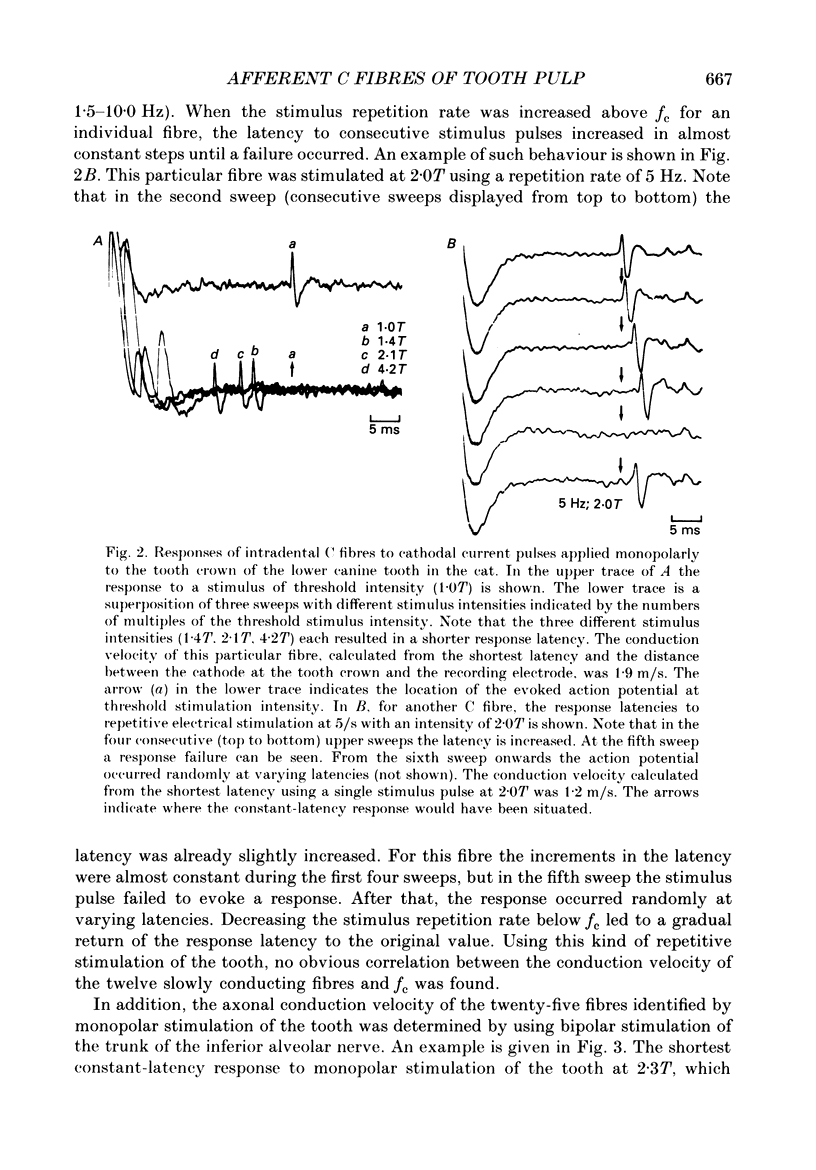
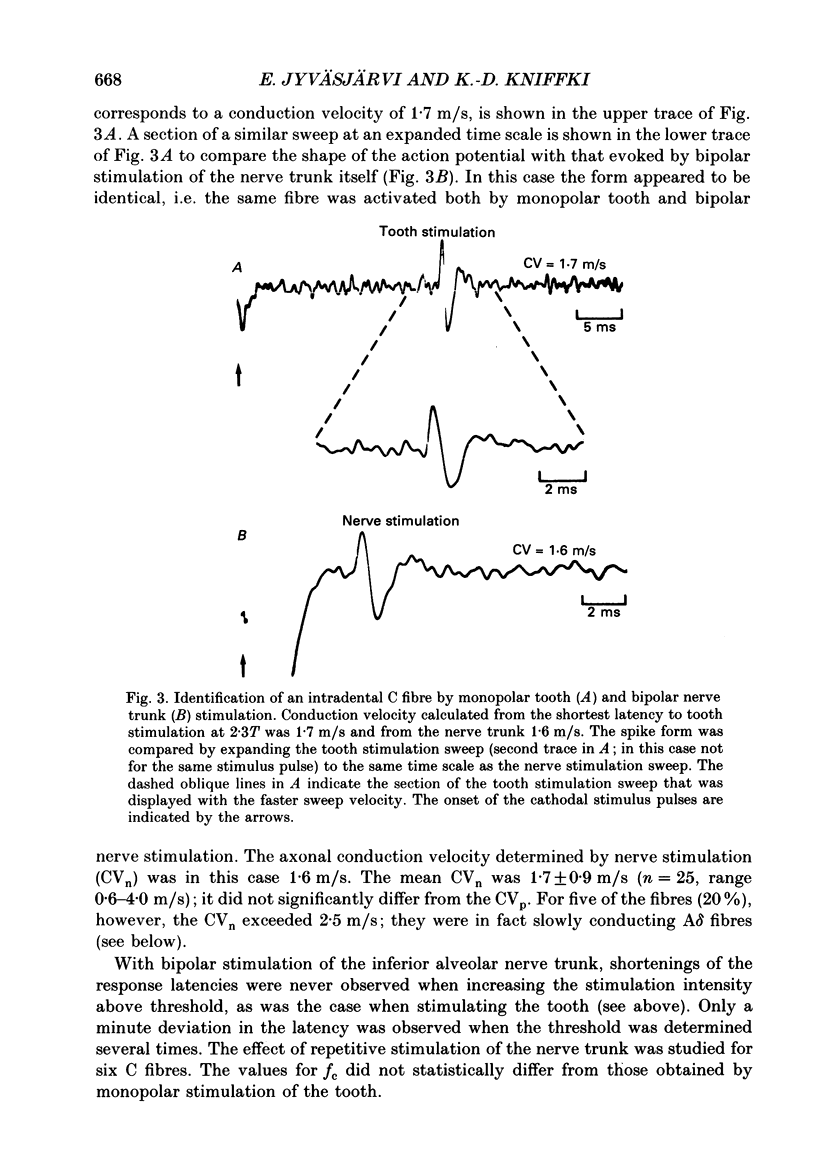
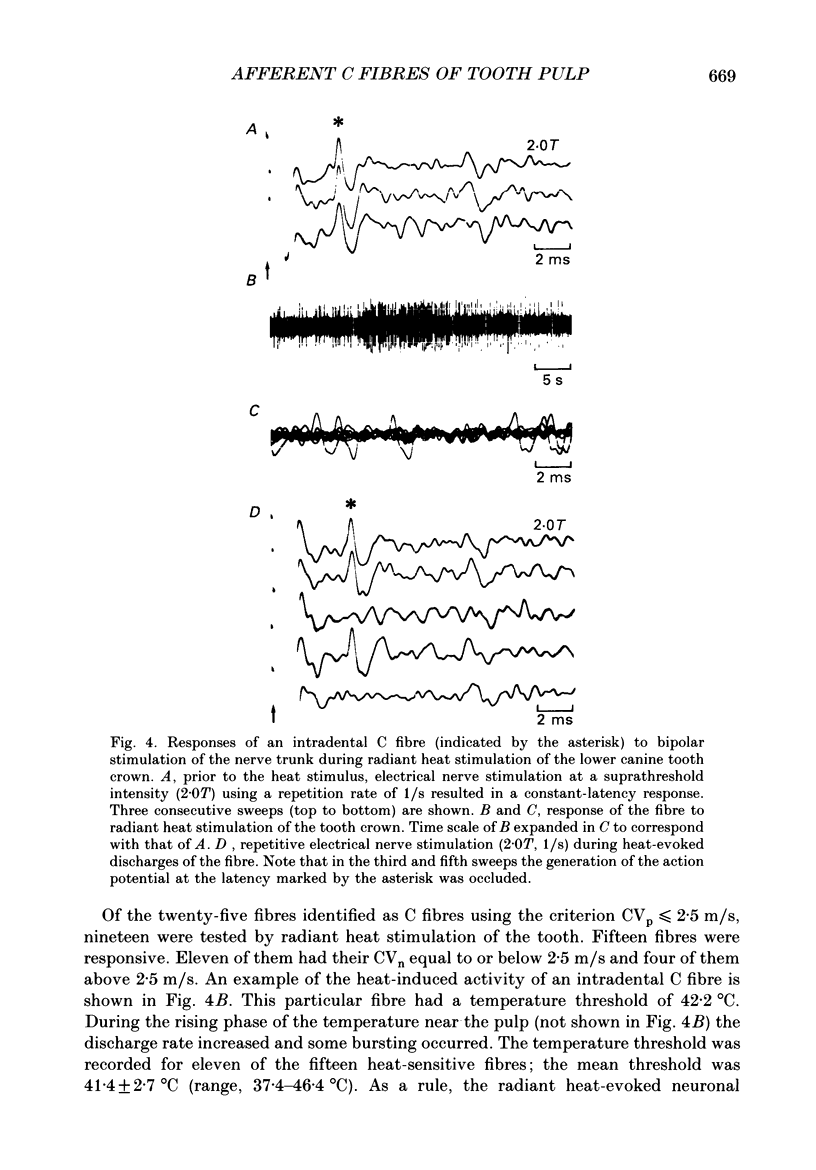




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Pearl G. S. C-Fiber activity in feline tooth pulp afferents. Exp Neurol. 1975 May;47(2):357–359. doi: 10.1016/0014-4886(75)90264-2. [DOI] [PubMed] [Google Scholar]
- Anderson K. V., Pearl G. S. Conduction velocities in afferent fibers from feline tooth pulp. Exp Neurol. 1974 Apr;43(1):281–283. doi: 10.1016/0014-4886(74)90147-2. [DOI] [PubMed] [Google Scholar]
- Arwill T., Edwall L., Lilja J., Olgart L., Svensson S. E. Ultrastructure of nerves in the dentinal-pulp border zone after sensory and autonomic nerve transection in the cat. Acta Odontol Scand. 1973;31(5):273–281. doi: 10.3109/00016357309002514. [DOI] [PubMed] [Google Scholar]
- Bessou P., Gauthier J., Pagès B. Mise en évidence de fibres afférentes du groupe C innervant la pulpe de la cannine, chez le chat. C R Seances Soc Biol Fil. 1970;164(8):1845–1850. [PubMed] [Google Scholar]
- Byers M. R. Dental sensory receptors. Int Rev Neurobiol. 1984;25:39–94. doi: 10.1016/s0074-7742(08)60677-7. [DOI] [PubMed] [Google Scholar]
- Cadden S. W., Lisney S. J., Matthews B. Thresholds to electrical stimulation of nerves in cat canine tooth-pulp with A beta-, A delta- and C-fibre conduction velocities. Brain Res. 1983 Feb 14;261(1):31–41. doi: 10.1016/0006-8993(83)91280-5. [DOI] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol. 1968 Dec;199(2):319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood F., Horiuchi H., Matthews B. Electrophysiological evidence on the types of nerve fibres excited by electrical stimulation of teeth with a pulp tester. Arch Oral Biol. 1972 Apr;17(4):701–709. doi: 10.1016/0003-9969(72)90196-3. [DOI] [PubMed] [Google Scholar]
- Hoffmeister B., Schendel K. Analyse markhaltiger und markloser Axone an den Zähnen des Unterkiefers der Katze. Dtsch Zahnarztl Z. 1986 Sep;41(9):863–867. [PubMed] [Google Scholar]
- Holland G. R. Fibre numbers and sizes in the inferior alveolar nerve of the cat. J Anat. 1978 Oct;127(Pt 2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Jiffry M. T. Afferent innervation of the rat incisor pulp. Exp Neurol. 1981 Jul;73(1):209–218. doi: 10.1016/0014-4886(81)90056-x. [DOI] [PubMed] [Google Scholar]
- Kerr F. W. The ultrastructure of the spinal tract of the trigeminal nerve and the substantia gelatinosa. Exp Neurol. 1966 Dec;16(4):359–376. doi: 10.1016/0014-4886(66)90104-x. [DOI] [PubMed] [Google Scholar]
- Lisney S. J. Some anatomical and electrophysiological properties of tooth-pulp afferents in the cat. J Physiol. 1978 Nov;284:19–36. doi: 10.1113/jphysiol.1978.sp012525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B., Holland G. R. Coupling between nerves in teeth. Brain Res. 1975 Nov 14;98(2):354–358. doi: 10.1016/0006-8993(75)90013-x. [DOI] [PubMed] [Google Scholar]
- Matthews B. Responses of intradental nerves to electrical and thermal stimulation of teeth in dogs. J Physiol. 1977 Jan;264(3):641–664. doi: 10.1113/jphysiol.1977.sp011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B., Robinson P. P. The course of post-ganglionic sympathetic fibres distributed with the trigeminal nerve in the cat. J Physiol. 1980 Jun;303:391–401. doi: 10.1113/jphysiol.1980.sp013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B., Searle B. N. Electrical stimulation of teeth. Pain. 1976 Sep;2(3):245–251. doi: 10.1016/0304-3959(76)90003-8. [DOI] [PubMed] [Google Scholar]
- Mumford J. M., Newton A. V. Zone of excitation when electrically stimulating human teeth. Arch Oral Biol. 1969 Dec;14(12):1383–1388. doi: 10.1016/0003-9969(69)90254-4. [DOI] [PubMed] [Google Scholar]
- Noga B. R., Holland G. R. Sympathetic innervation at the apex of the cat's canine tooth - a quantitative analysis. Anat Anz. 1983;153(2):137–148. [PubMed] [Google Scholar]
- Närhi M., Jyväsjärvi E., Hirvonen T., Huopaniemi T. Activation of heat-sensitive nerve fibres in the dental pulp of the cat. Pain. 1982;14(4):317–326. doi: 10.1016/0304-3959(82)90140-3. [DOI] [PubMed] [Google Scholar]
- Närhi M., Virtanen A., Huopaniemi T., Hirvonen T. Conduction velocities of single pulp nerve fibre units in the cat. Acta Physiol Scand. 1982 Nov;116(3):209–213. doi: 10.1111/j.1748-1716.1982.tb07132.x. [DOI] [PubMed] [Google Scholar]
- Reader A., Foreman D. W. An ultrastructural quantitative investigation of human intradental innervation.. J Endod. 1981 Nov;7(11):493–499. doi: 10.1016/S0099-2399(81)80111-2. [DOI] [PubMed] [Google Scholar]
- Virtanen A. S. Electrical stimulation of pulp nerves--comparison of monopolar and bipolar electrode coupling. Pain. 1985 Nov;23(3):279–288. doi: 10.1016/0304-3959(85)90106-X. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Närhi M., Huopaniemi T., Hirvonen T. Thresholds of intradental A- and C-nerve fibres in the cat to electrical current pulses of different duration. Acta Physiol Scand. 1983 Dec;119(4):393–398. doi: 10.1111/j.1748-1716.1983.tb07355.x. [DOI] [PubMed] [Google Scholar]
- Young R. F., King R. B. Fiber spectrum of the trigeminal sensory root of the baboon determined by electron microscopy. J Neurosurg. 1973 Jan;38(1):65–72. doi: 10.3171/jns.1973.38.1.0065. [DOI] [PubMed] [Google Scholar]


