Abstract
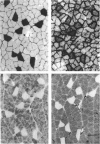
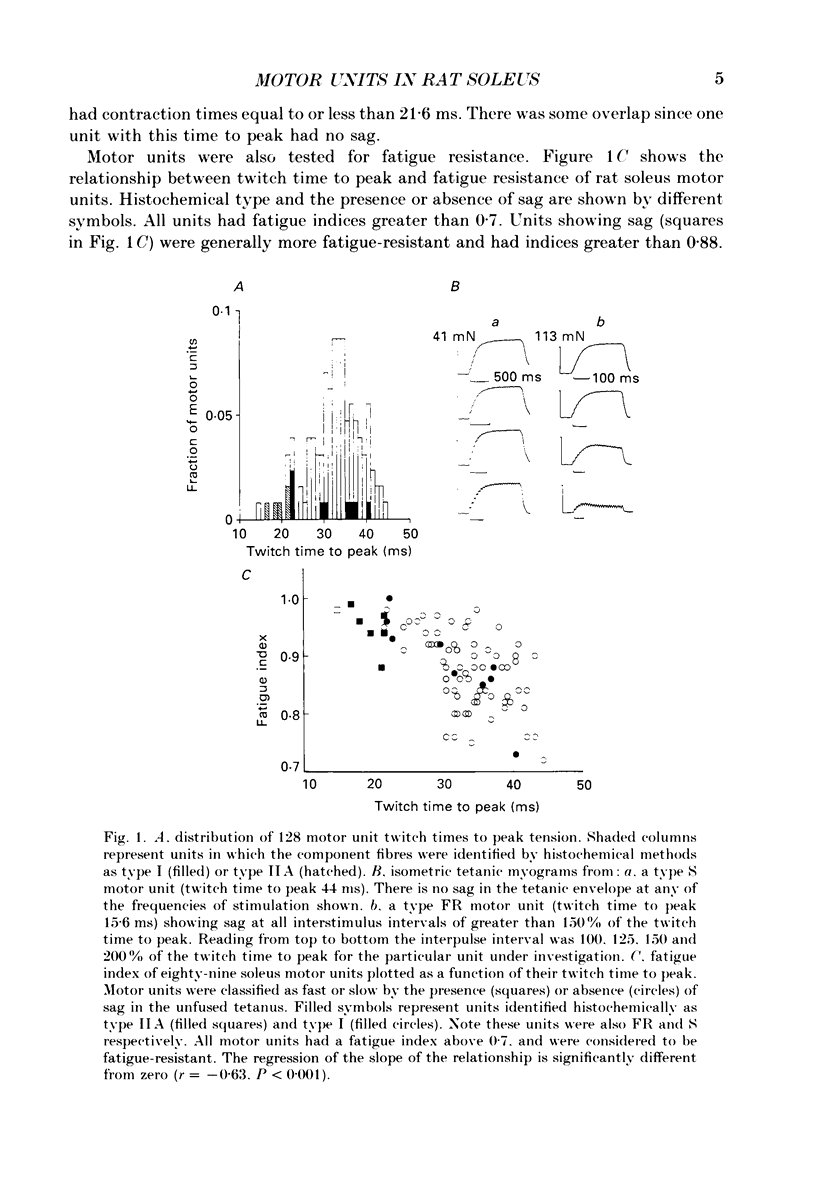
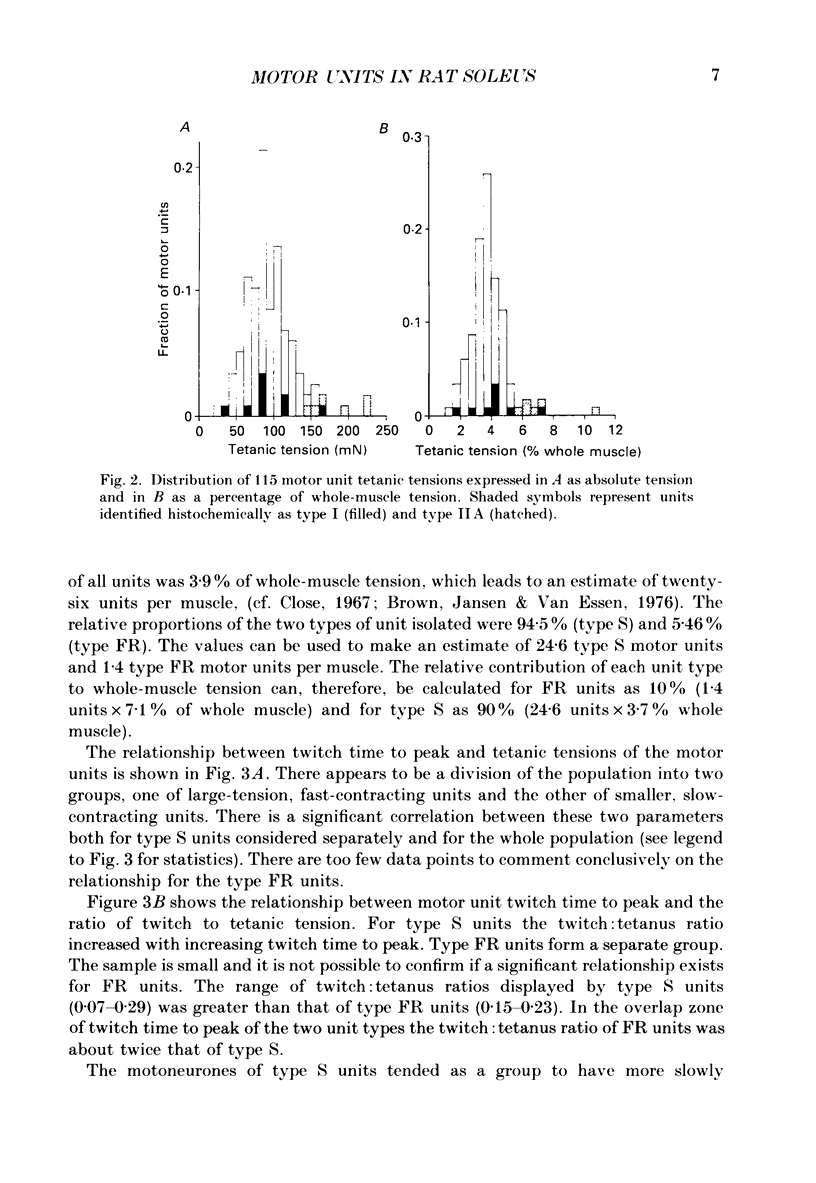
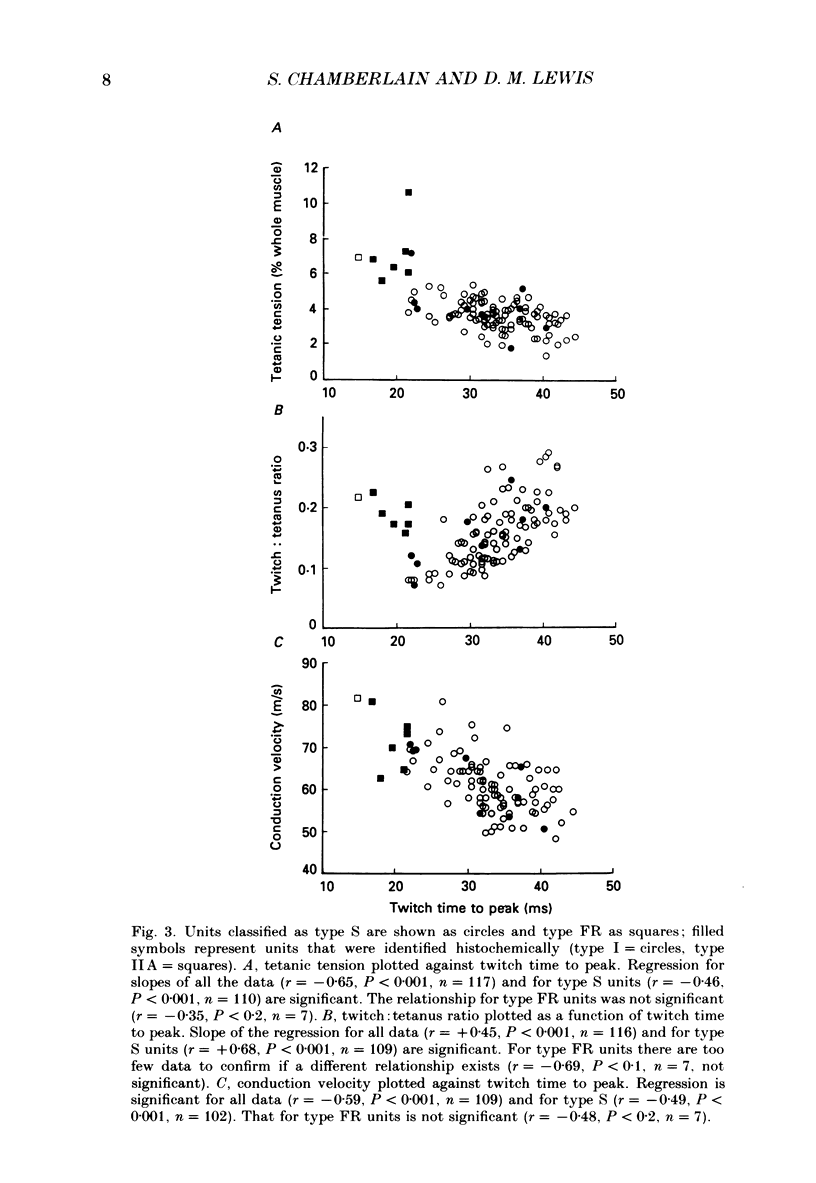
1. Physiological properties of motor units in the soleus muscle were studied in anaesthetized rats using ventral root splitting to isolate single units. 2. Motor unit types were classified by the same criteria used to classify cat hindlimb motor units into types FR (fast-twitch, fatigue-resistant) and type S (slow-twitch, fatigue-resistant). Type FR units were estimated to generate 10% of whole-muscle tension and type S 90%. All FR units showed sag in the unfused tetanus at frequencies with interpulse intervals greater than 175% of twitch time to peak, but not at 125% (Burke, Levine, Tsairis & Zajac, 1973). 3. The muscle fibres belonging to twelve single motor units were depleted of glycogen by prolonged stimulation, permitting analysis of their histochemical profiles. Type FR units were found to consist of type IIA muscle fibres and type S units of type I muscle fibres. 4. Direct determinations were made of fibre area, innervation ratio (number of muscle fibres supplied by one motoneurone) and hence specific tension (tetanic tension generated per unit cross-sectional area) of individually identified motor units. Motoneurons were found to innervate between 84 and 178 muscle fibres (mean 110) in type S units and between 126 and 161 fibres in type FR units (mean 142). Fibre areas were larger for type FR units and there was a significant difference in specific tension of the two unit types (type S lower). 5. Indirect estimates of innervation ratio and specific tension were obtained from counts of muscle fibre types, and relative frequencies of motor unit types in the soleus unit pool. Observations agreed well with results of direct measurements. 6. The evidence provided suggests that differences in tension generated by type FR and S units in rat soleus muscle are primarily due to differences in innervation ratio and fibre area, with a small contribution from differences in specific tension.
Full text
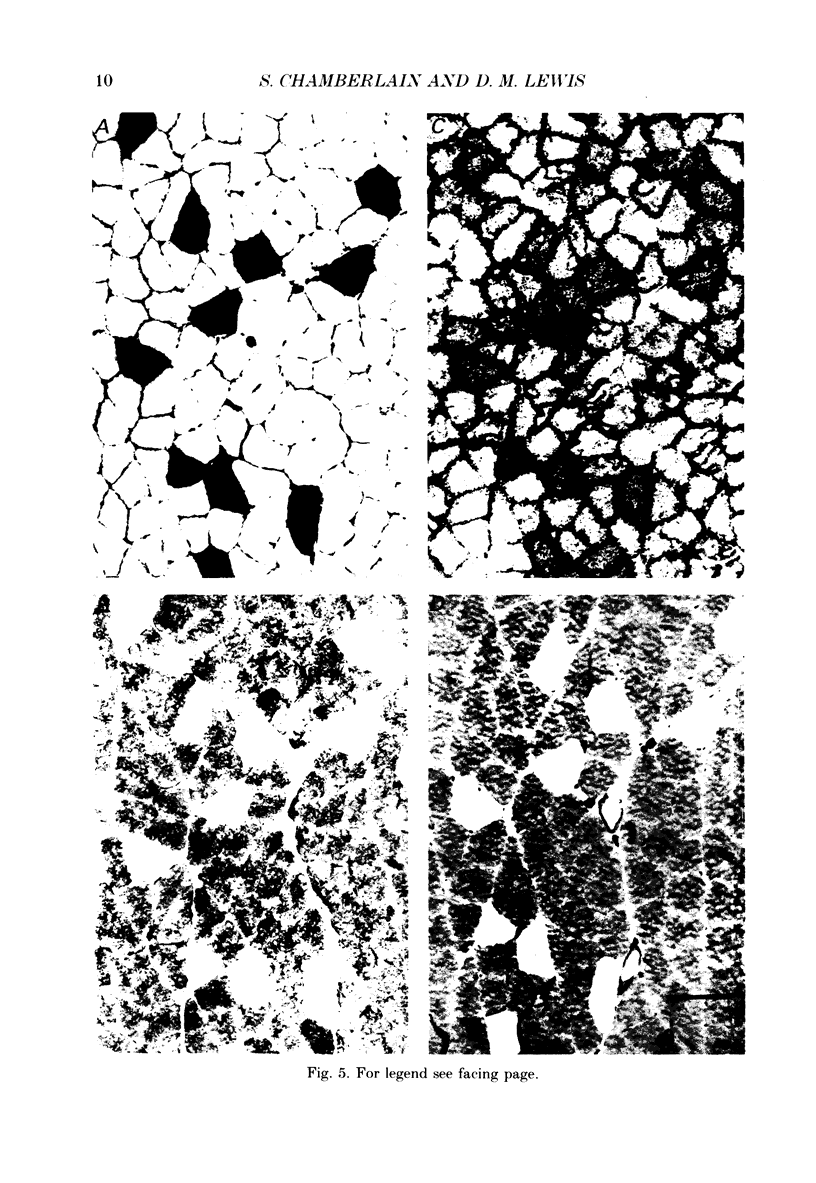
PDF






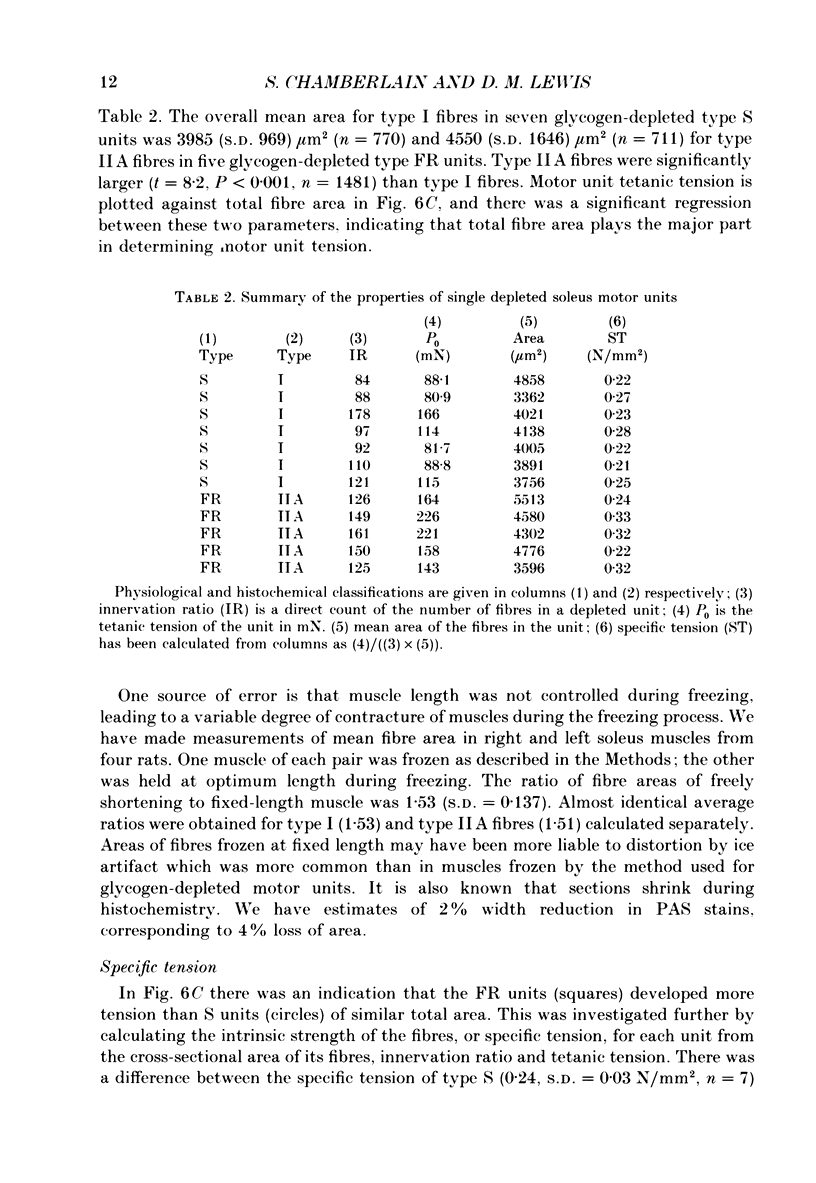
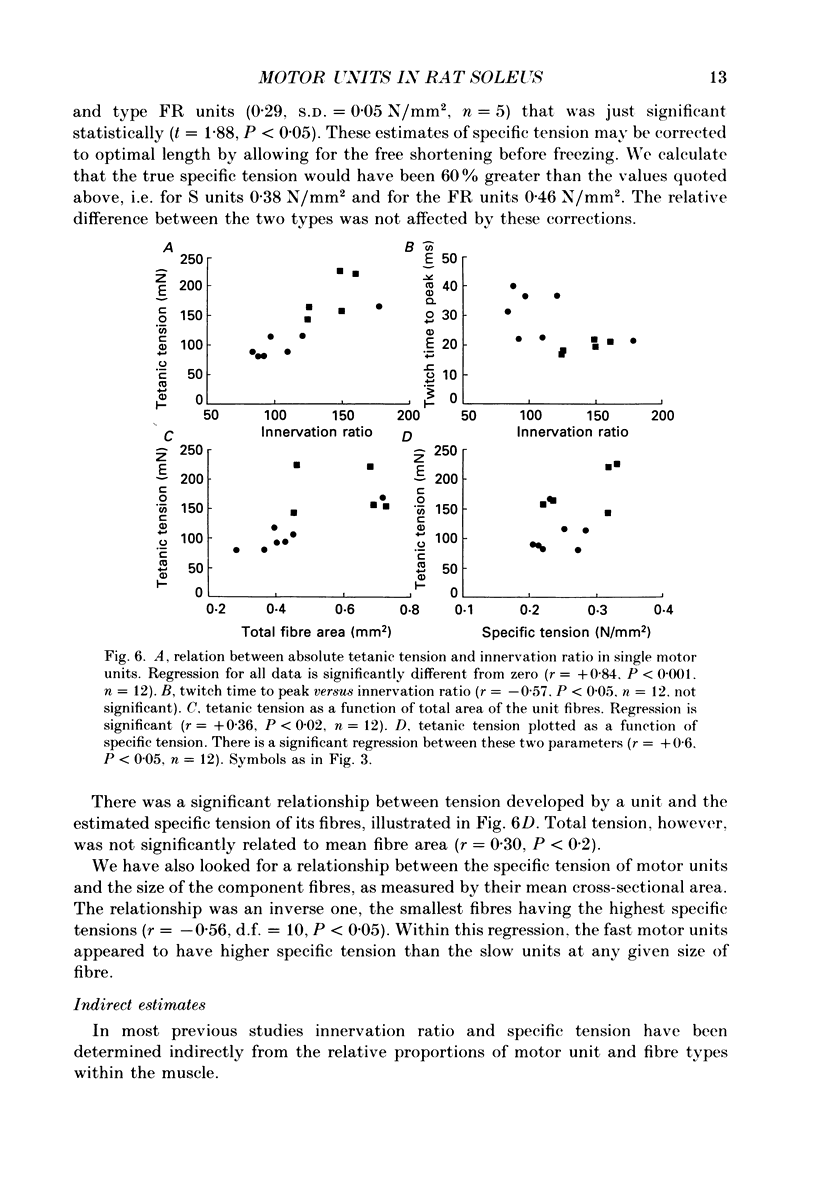








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Amood W. S., Finol H. J., Lewis D. M. Chronic stimulation modifies the isotonic shortening velocity of denervated rat slow-twitch muscle. Proc R Soc Lond B Biol Sci. 1986 Jun 23;228(1250):43–58. doi: 10.1098/rspb.1986.0039. [DOI] [PubMed] [Google Scholar]
- Bagust J. Relationships between motor nerve conduction velocities and motor unit contraction characteristics in a slow twitch muscle of the cat. J Physiol. 1974 Apr;238(2):269–278. doi: 10.1113/jphysiol.1974.sp010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Burke R. E., O'Donovan M. J., Toop J., Hodgson J. A. Motor-unit organization in flexor digitorum longus muscle of the cat. J Neurophysiol. 1982 Jun;47(6):1108–1125. doi: 10.1152/jn.1982.47.6.1108. [DOI] [PubMed] [Google Scholar]
- Edjtehadi G. D., Lewis D. M. Histochemical reactions of fibres in a fast twitch muscle of the cat. J Physiol. 1979 Feb;287:439–453. doi: 10.1113/jphysiol.1979.sp012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmubarak M. H., Ranatunga K. W. Differentiation of fast and slow muscles in the rat after neonatal denervation: a physiological study. J Muscle Res Cell Motil. 1988 Jun;9(3):219–232. doi: 10.1007/BF01773892. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Stonnington H. H. Trophic functions of the neuron. II. Denervation and regulation of muscle. Morphological effects of denervation of muscle. A quantitative ultrastructural study. Ann N Y Acad Sci. 1974 Mar 22;228(0):68–88. doi: 10.1111/j.1749-6632.1974.tb20503.x. [DOI] [PubMed] [Google Scholar]
- Finol H. J., Lewis D. M., Owens R. The effects of denervation on contractile properties or rat skeletal muscle. J Physiol. 1981;319:81–92. doi: 10.1113/jphysiol.1981.sp013893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P. F., Olha A. E. Contractile and electromyographic characteristics of rat plantaris motor unit types during fatigue in situ. J Physiol. 1987 Apr;385:13–34. doi: 10.1113/jphysiol.1987.sp016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977 Aug;19(2):103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- Kean C. J., Lewis D. M., McGarrick J. D. Dynamic properties of denervated fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):103–113. doi: 10.1113/jphysiol.1974.sp010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- Owens R. An isolated perfused rat skeletal muscle preparation [proceedings]. J Physiol. 1978 Apr;277:9P–9P. [PubMed] [Google Scholar]
- Ranatunga K. W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984 Jun;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Amood W. S., Pope R. A comparison of the structural features of muscle fibres from a fast- and a slow-twitch muscle of the pelvic limb of the cat. J Anat. 1972 Oct;113(Pt 1):49–60. [PMC free article] [PubMed] [Google Scholar]