Abstract
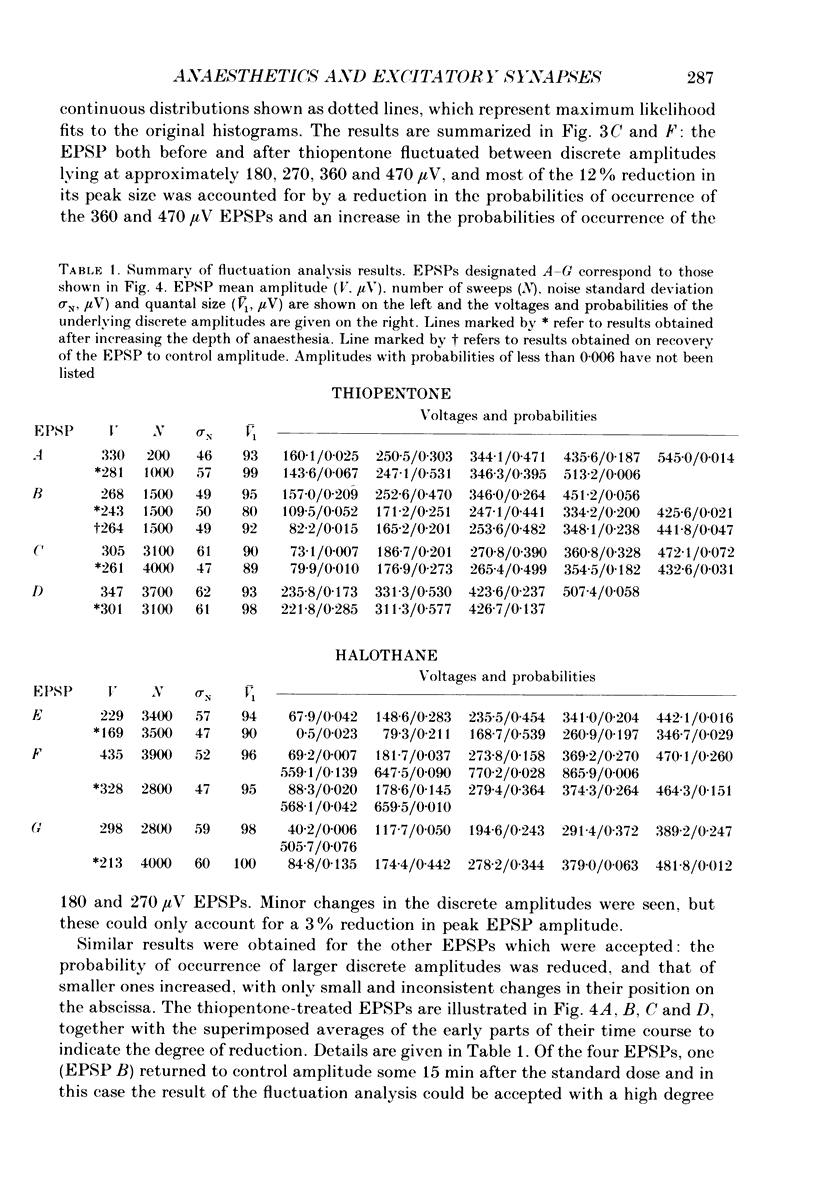
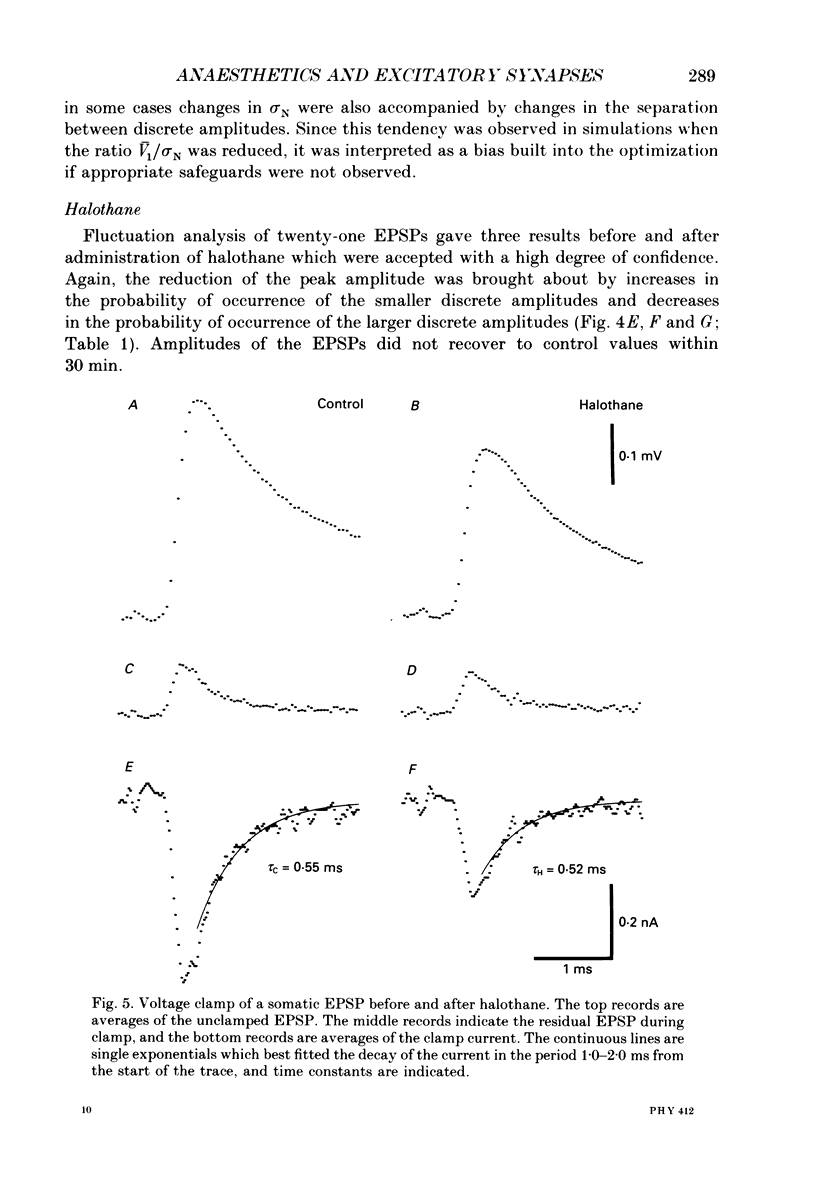
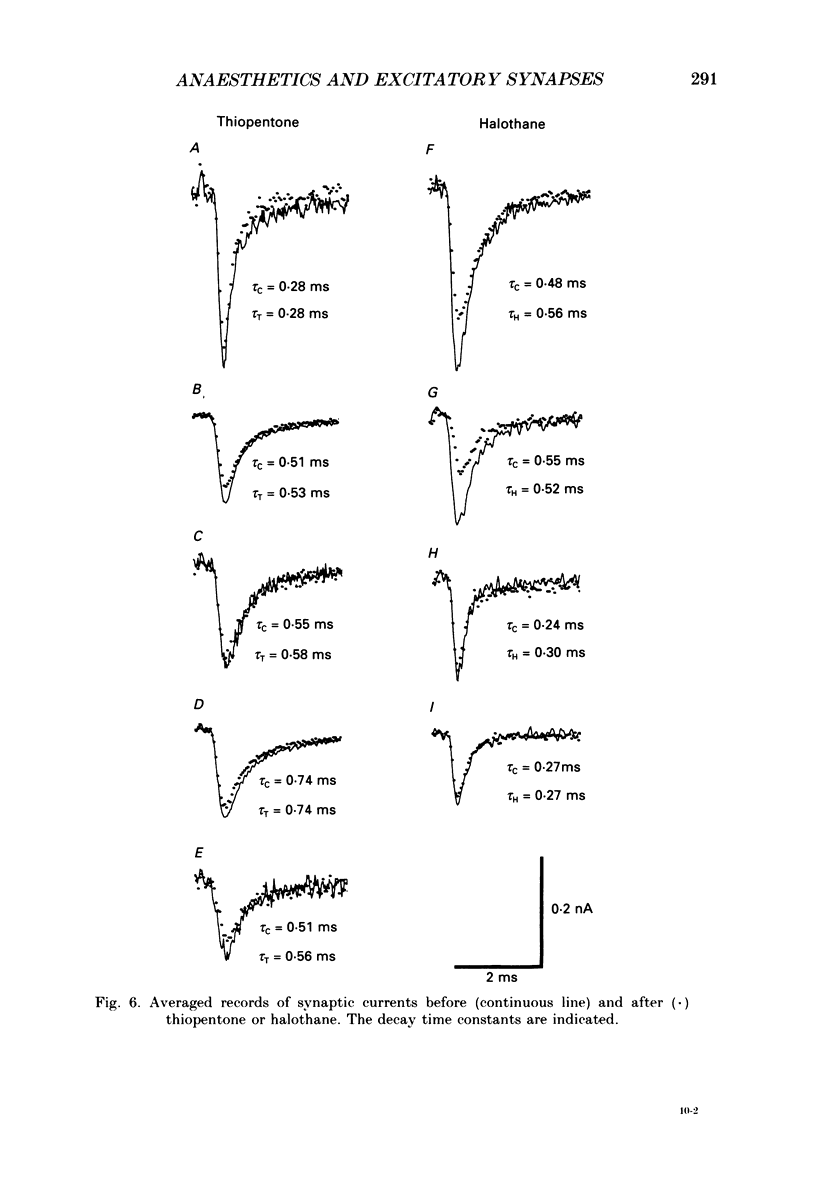
1. The effects of thiopentone and halothane on excitatory synaptic transmission at group Ia afferent synapses on lumbosacral motoneurones were studied in the anaesthetized or decerebrate cat. 2. Thiopentone (10 mg kg-1) infused on a background of light pentobarbitone anaesthesia caused a decrease in single-fibre monosynaptic group Ia excitatory postsynaptic potentials (EPSPs) of between 0 and 24%. A step increase in inspired halothane concentration in the range 0.7-0.9% produced a decrease in EPSP amplitude of between 0 and 31%. These effects were reversible when the anaesthetic level was reduced. 3. Fluctuation analysis of selected single-fibre group Ia EPSPs revealed that these effects could be accounted for by a decrease in the probability of occurrence of EPSPs of larger amplitude, and an increase in the probability of occurrence of EPSPs of smaller amplitude. The mean separation between discrete amplitudes was not altered by either anaesthetic agent. 4. EPSPs whose time course indicated a somatic site of origin were voltage clamped to study the effect of the anaesthetics on the time course of the synaptic currents. Neither thiopentone nor halothane produced a consistent effect on the time constant of decay of the current, although they both depressed its peak amplitude. 5. The results are interpreted as indicating a presynaptic site of action of both anaesthetics at the concentrations studied: the probability of release of neurotransmitter is reduced, without any detectable change in the mean duration of the postsynaptic conductance increase. These findings are discussed in relation to the mechanisms of action of anaesthetics on exocytosis and presynaptic inhibition.
Full text
PDF




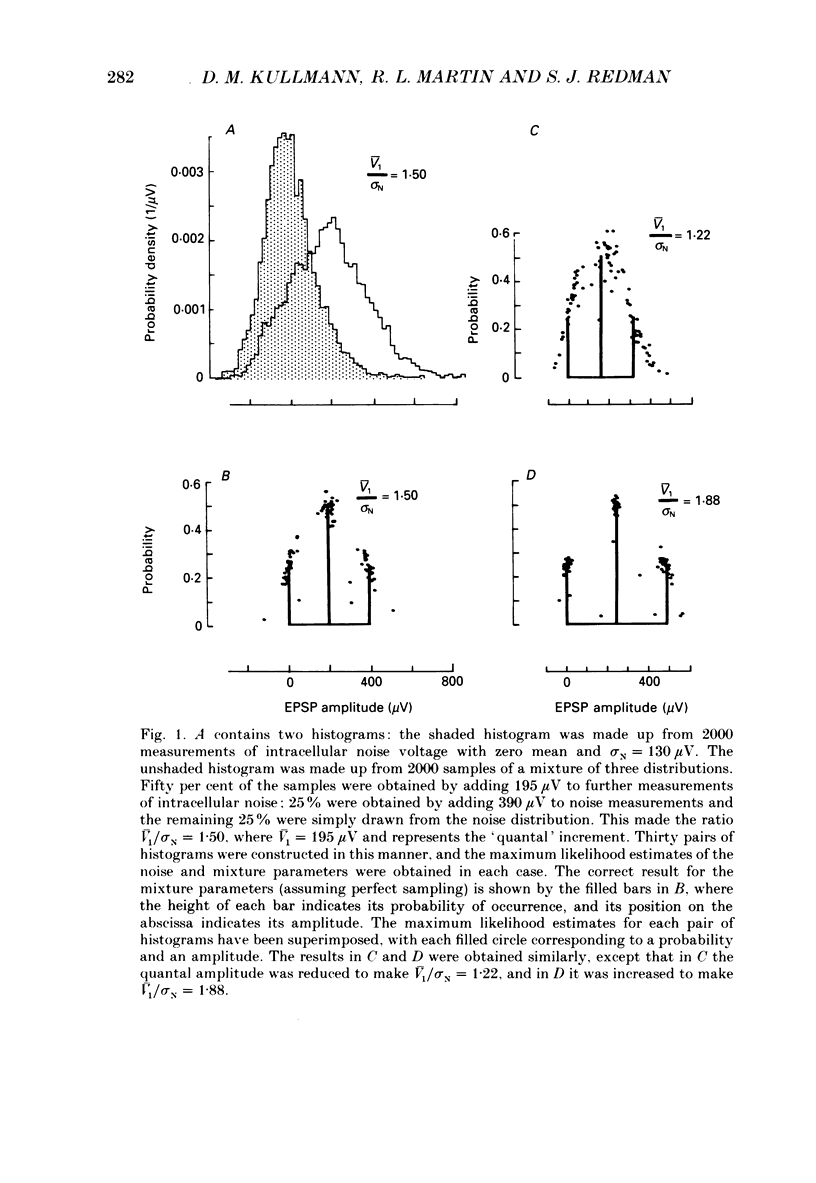

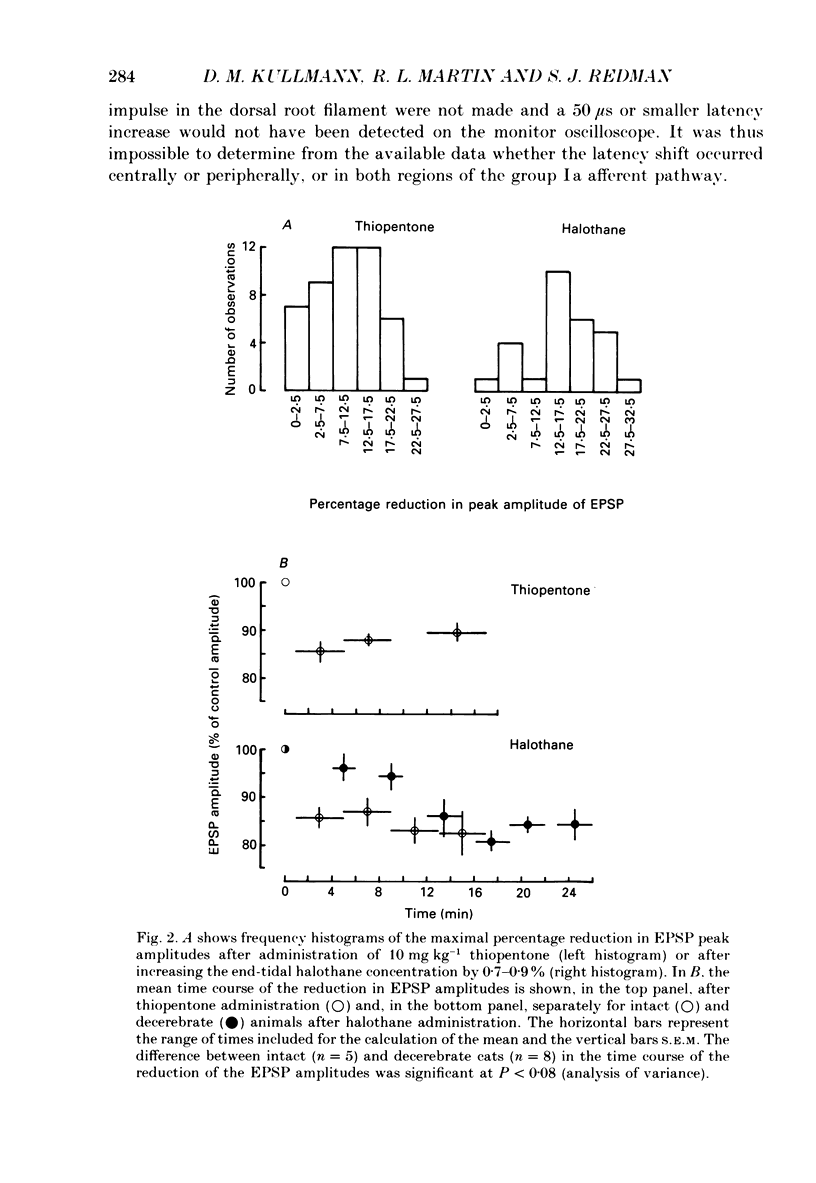
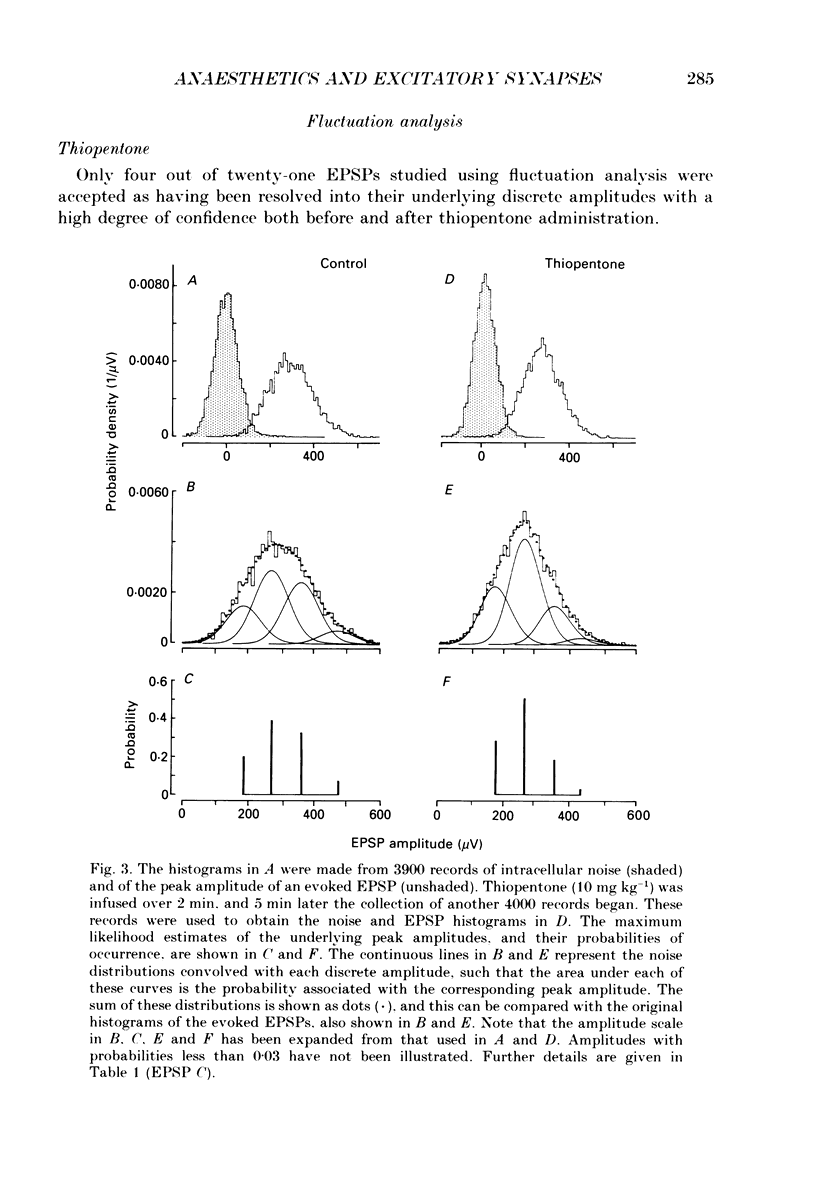
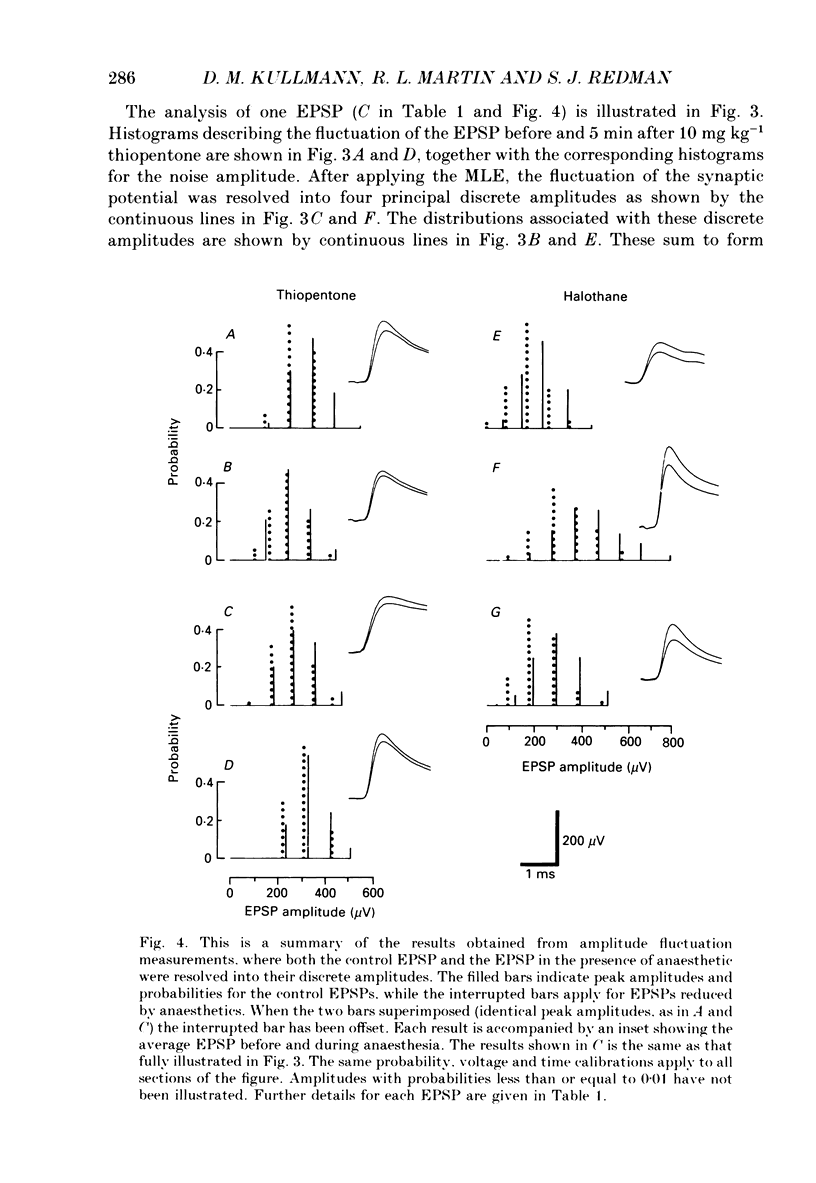







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., BERNSTEIN E., MARK L. C. The role of body fat in limiting the duration of action of thiopental. J Pharmacol Exp Ther. 1952 Aug;105(4):421–426. [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):319–327. doi: 10.1098/rspb.1979.0108. [DOI] [PubMed] [Google Scholar]
- CHENOWETH M. B., ROBERTSON D. N., ERLEY D. S., GOLHKE R. Blood and tissue levels of ether, chloroform, halothane and methoxyflurane in dogs. Anesthesiology. 1962 Jan-Feb;23:101–106. doi: 10.1097/00000542-196201000-00016. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Forsythe I. D., Redman S. J. Presynaptic inhibition of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia axons. J Physiol. 1987 Feb;383:153–169. doi: 10.1113/jphysiol.1987.sp016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Nelson P. G., Redman S. J. Intracellular tetraethylammonium ions enhance group Ia excitatory post-synaptic potentials evoked in cat motoneurones. J Physiol. 1986 Aug;377:267–282. doi: 10.1113/jphysiol.1986.sp016186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R. Pharmacological studies on feline Betz cells. J Physiol. 1966 Sep;186(1):121–138. doi: 10.1113/jphysiol.1966.sp008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Wilson V. J., Malik R. The effect of GABA on the terminations of vestibulospinal neurons in the cat spinal cord. Brain Res. 1984 Mar 19;295(2):372–375. doi: 10.1016/0006-8993(84)90989-2. [DOI] [PubMed] [Google Scholar]
- Cutler R. W., Markowitz D., Dudzinski D. S. The effect of barbiturates on (3H)GABA transport in rat cerebral cortex slices. Brain Res. 1974 Dec 6;81(2):189–197. doi: 10.1016/0006-8993(74)90935-4. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. A shielded microelectrode suitable for single-electrode voltage clamping of neurones in the CNS. J Neurosci Methods. 1983 Sep;9(1):23–29. doi: 10.1016/0165-0270(83)90105-x. [DOI] [PubMed] [Google Scholar]
- Frank M., Savege T. M., Leigh M., Greenwood J., Holly J. M. Comparison of the cerebral function monitor and plasma concentrations of thiopentone and alphaxalone during total i.v. anaesthesia with repeated bolus doses of thiopentone and althesin. Br J Anaesth. 1982 Jun;54(6):609–616. doi: 10.1093/bja/54.6.609. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Effects of procaine, pentobarbital and halothane on synaptic transmission in the central nervous system. J Pharmacol Exp Ther. 1969 Oct;169(2):185–195. [PubMed] [Google Scholar]
- Harrison P. J., Jack J. J., Kullmann D. M. Monosynaptic EPSPs in cat lumbosacral motoneurones from group Ia afferents and fibres descending in the spinal cord. J Physiol. 1989 May;412:43–63. doi: 10.1113/jphysiol.1989.sp017603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Tolhurst D. J. Recovering the parameters of finite mixtures of normal distributions from a noisy record: an empirical comparison of different estimating procedures. J Neurosci Methods. 1983 Aug;8(4):309–333. doi: 10.1016/0165-0270(83)90090-0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E. K., QUILLIAM J. P. EFFECTS OF CENTRAL DEPRESSANT DRUGS UPON ACETYLCHOLINE RELEASE. Br J Pharmacol Chemother. 1964 Apr;22:415–440. doi: 10.1111/j.1476-5381.1964.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Anticonvulsant and anesthetic barbiturates: different postsynaptic actions in cultured mammalian neurons. Neurology. 1979 Apr;29(4):432–447. doi: 10.1212/wnl.29.4.432. [DOI] [PubMed] [Google Scholar]
- Morgan D. J., Crankshaw D. P., Prideaux P. R., Chan H. N., Boyd M. D. Thiopentone levels during cardiopulmonary bypass. Changes in plasma protein binding during continuous infusion. Anaesthesia. 1986 Jan;41(1):4–10. doi: 10.1111/j.1365-2044.1986.tb12695.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pentobarbital: action on frog motoneurons. Brain Res. 1975 Oct 10;96(1):119–123. doi: 10.1016/0006-8993(75)90582-x. [DOI] [PubMed] [Google Scholar]
- Pocock G., Richards C. D. The action of pentobarbitone on stimulus-secretion coupling in adrenal chromaffin cells. Br J Pharmacol. 1987 Jan;90(1):71–80. doi: 10.1111/j.1476-5381.1987.tb16826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. Actions of general anaesthetics on synaptic transmission in the CNS. Br J Anaesth. 1983 Mar;55(3):201–207. doi: 10.1093/bja/55.3.201. [DOI] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of halothane anaesthesia. J Physiol. 1973 Sep;233(2):439–456. doi: 10.1113/jphysiol.1973.sp010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Smaje J. C. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976 Nov;58(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- SOMJEN G. G., GILL M. The mechanism of the blockade of synaptic transmission in the mammalian spinal cord by diethyl ether and by thiopental. J Pharmacol Exp Ther. 1963 Apr;140:19–30. [PubMed] [Google Scholar]
- Sawada S., Yamamoto C. Blocking action of pentobarbital on receptors for excitatory amino acids in the guinea pig hippocampus. Exp Brain Res. 1985;59(2):226–231. doi: 10.1007/BF00230901. [DOI] [PubMed] [Google Scholar]
- Staiman A., Seeman P. The impulse-blocking concentrations of anesthetics, alcohols, anticonvulsants, barbiturates, and narcotics on phrenic and sciatic nerves. Can J Physiol Pharmacol. 1974 Jun;52(3):535–550. doi: 10.1139/y74-071. [DOI] [PubMed] [Google Scholar]
- Takenoshita M., Takahashi T. Mechanisms of halothane action on synaptic transmission in motoneurons of the newborn rat spinal cord in vitro. Brain Res. 1987 Feb 3;402(2):303–310. doi: 10.1016/0006-8993(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Enhancement of GABA binding by pentobarbitone. Neurosci Lett. 1980 Jul;18(3):323–327. doi: 10.1016/0304-3940(80)90305-5. [DOI] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Pharmacology of barbiturates: electrophysiological and neurochemical studies. Int Rev Neurobiol. 1983;24:15–49. doi: 10.1016/s0074-7742(08)60219-6. [DOI] [PubMed] [Google Scholar]
- Yashima N., Wada A., Izumi F. Halothane inhibits the cholinergic-receptor-mediated influx of calcium in primary culture of bovine adrenal medulla cells. Anesthesiology. 1986 Apr;64(4):466–472. doi: 10.1097/00000542-198604000-00009. [DOI] [PubMed] [Google Scholar]
- Zorychta E., Capek R. Depression of spinal monosynaptic transmission by diethyl ether: quantal analysis of unitary synaptic potentials. J Pharmacol Exp Ther. 1978 Dec;207(3):825–836. [PubMed] [Google Scholar]


