Abstract
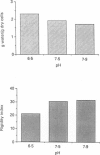
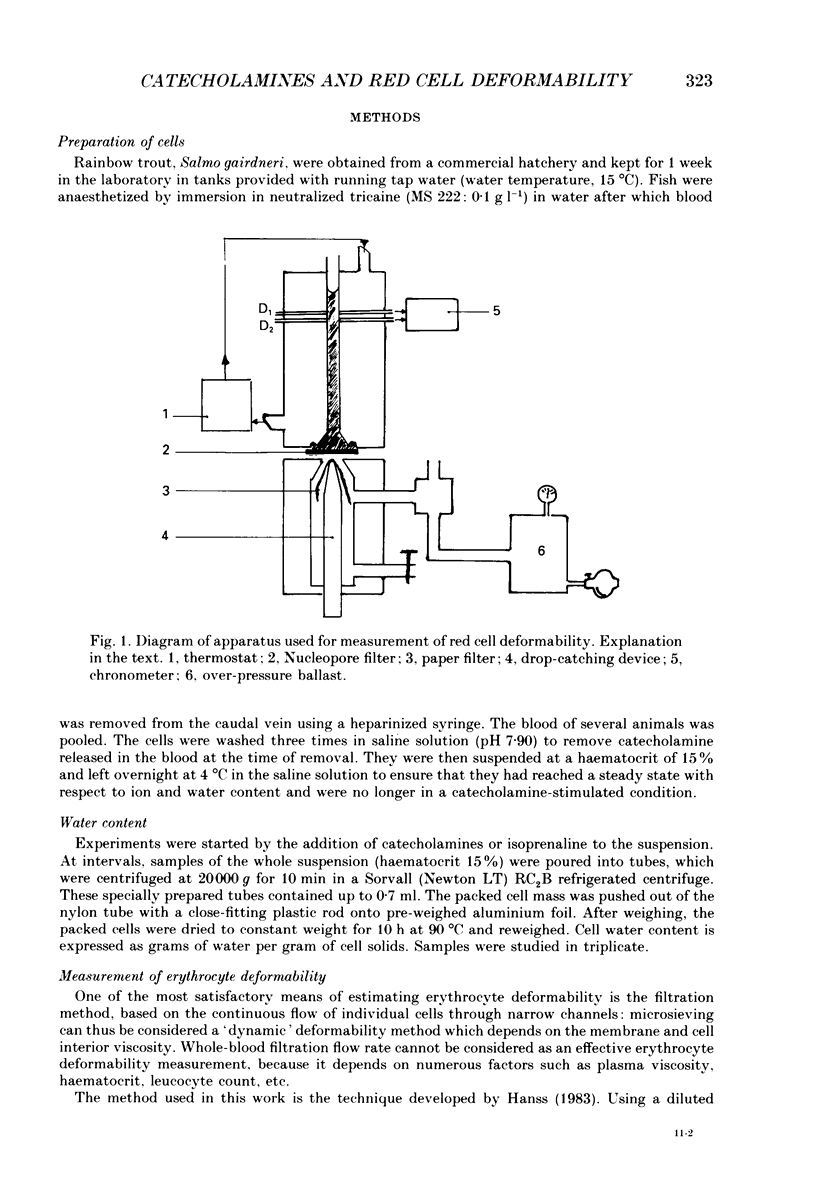
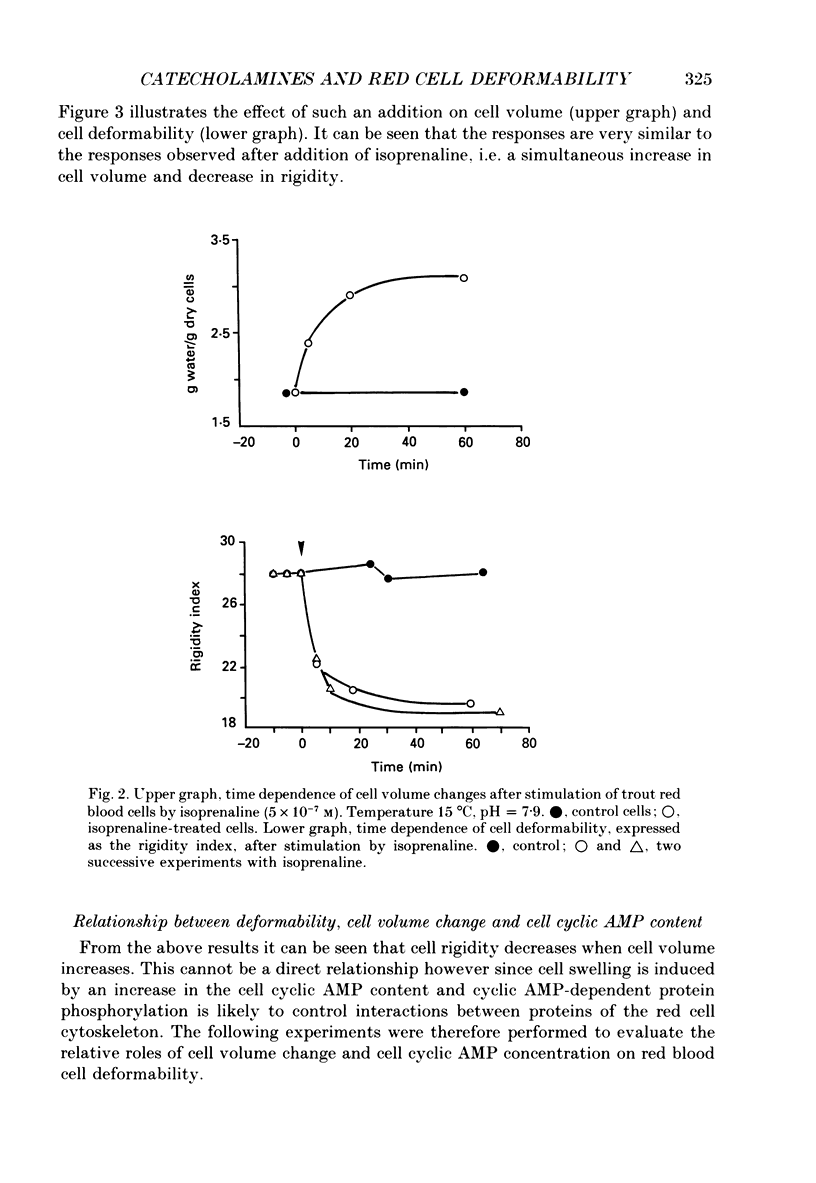
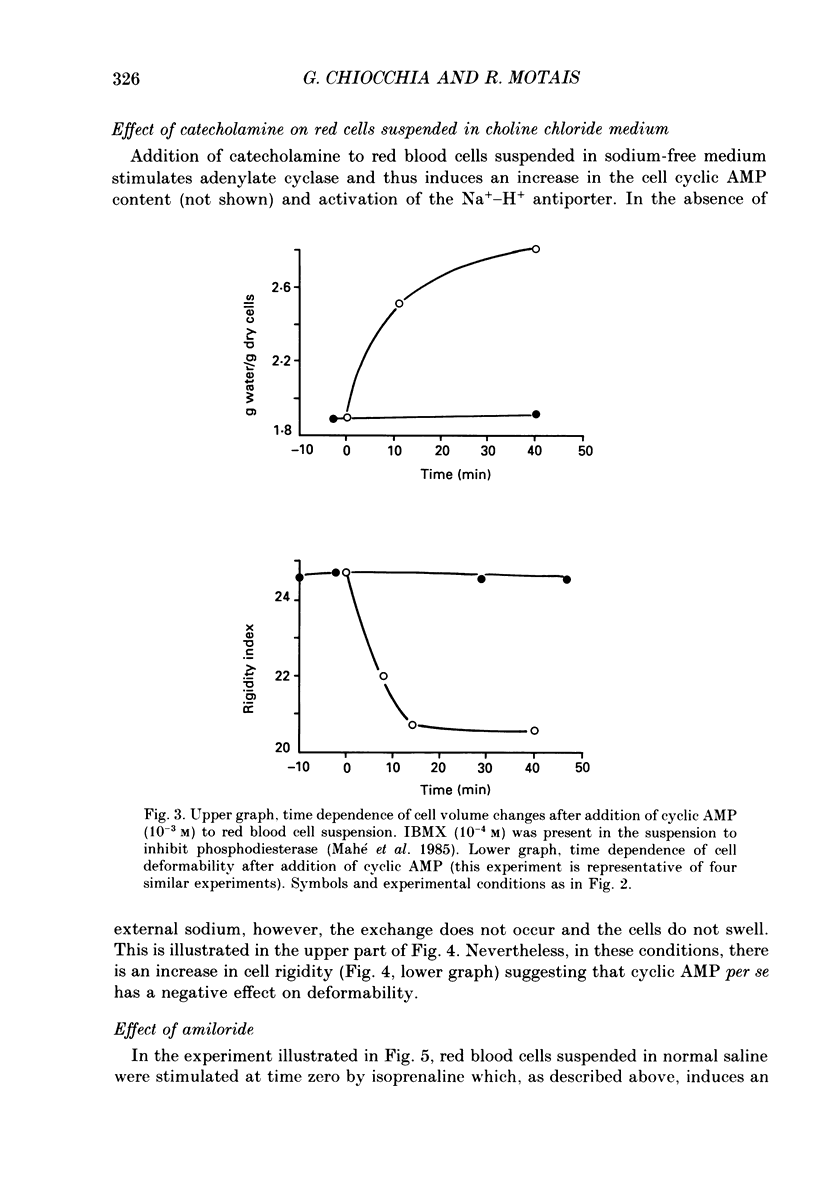
1. In the presence of catecholamine the nucleated red blood cells of trout show a large increase in cell volume as a result of an accumulation of sodium and chloride due to activation of an amiloride-sensitive, cyclic AMP-dependent Na+-H+ exchanger allowing Na+ to enter in exchange for internal H+. 2. The activation of this cyclic AMP-dependent Na+-H+ exchange is considered to be involved in an adaptive response to hypoxia by increasing the oxygen-carrying capacity of erythrocytes. But cell swelling could increase resistance to blood flow and thus impair the expected physiological advantages for oxygen transport. The effect of catecholamine on the deformability properties of the red blood cells has been studied by measuring the rate at which blood flows through a Nucleopore filter (5 microM). 3. The results show that stimulation by catecholamine in fact increases the erythrocyte deformability, a response which must favour the supply of oxygen at the tissue level. 4. Hormonal stimulation increases the cellular cyclic AMP content (and cyclic AMP-dependent phosphorylation of cytoskeleton proteins could influence cell deformability) and the cell volume. It has been shown that when cellular cyclic AMP content is increased under conditions where the cell cannot swell, the erythrocyte becomes more rigid and not more deformable. Conversely the results show a systematic coincidence between cell swelling and deformability increase. The precise way in which volume change and deformability are interrelated needs more study.
Full text
PDF


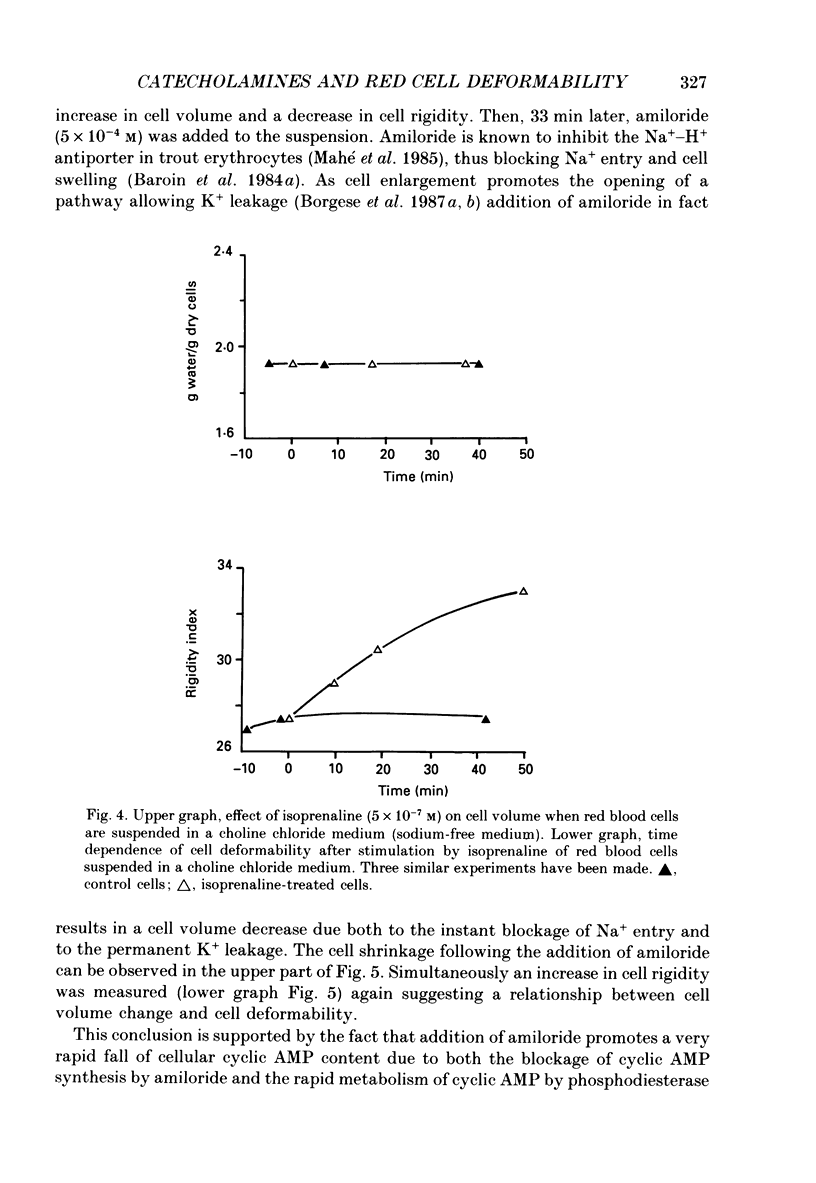
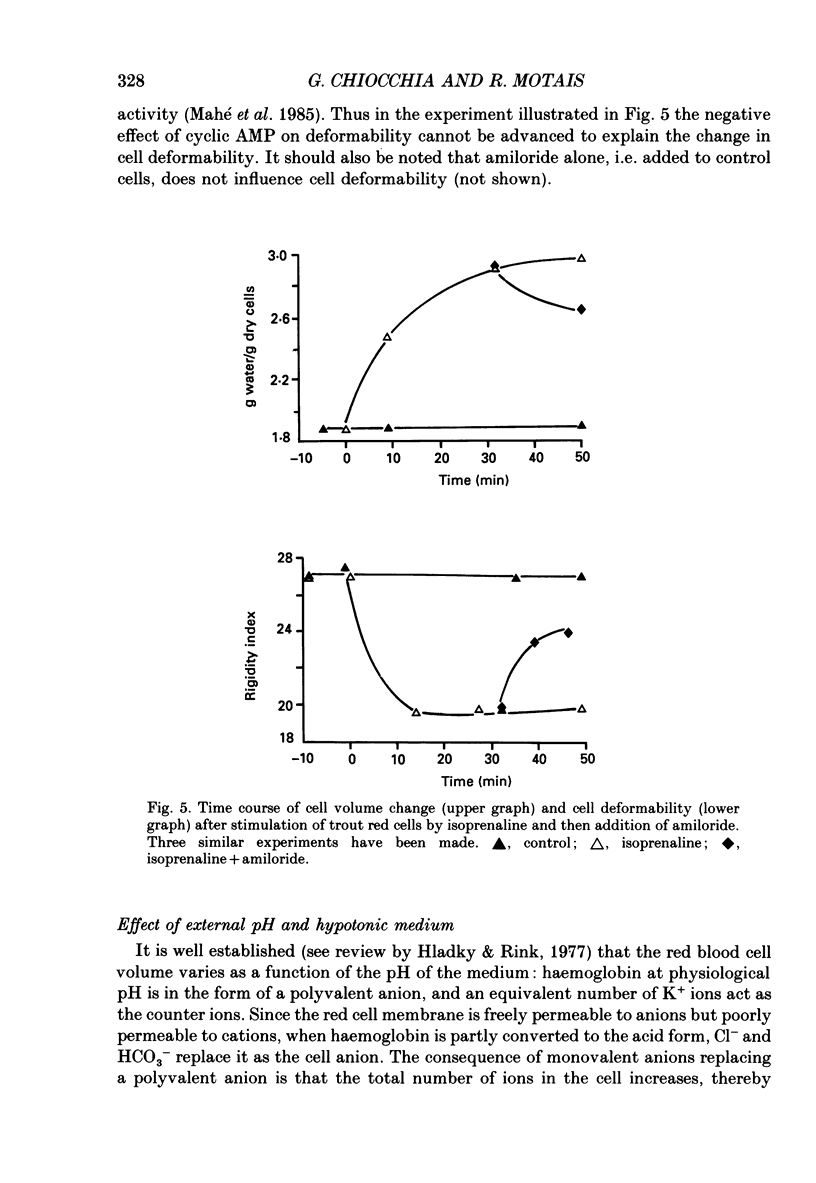
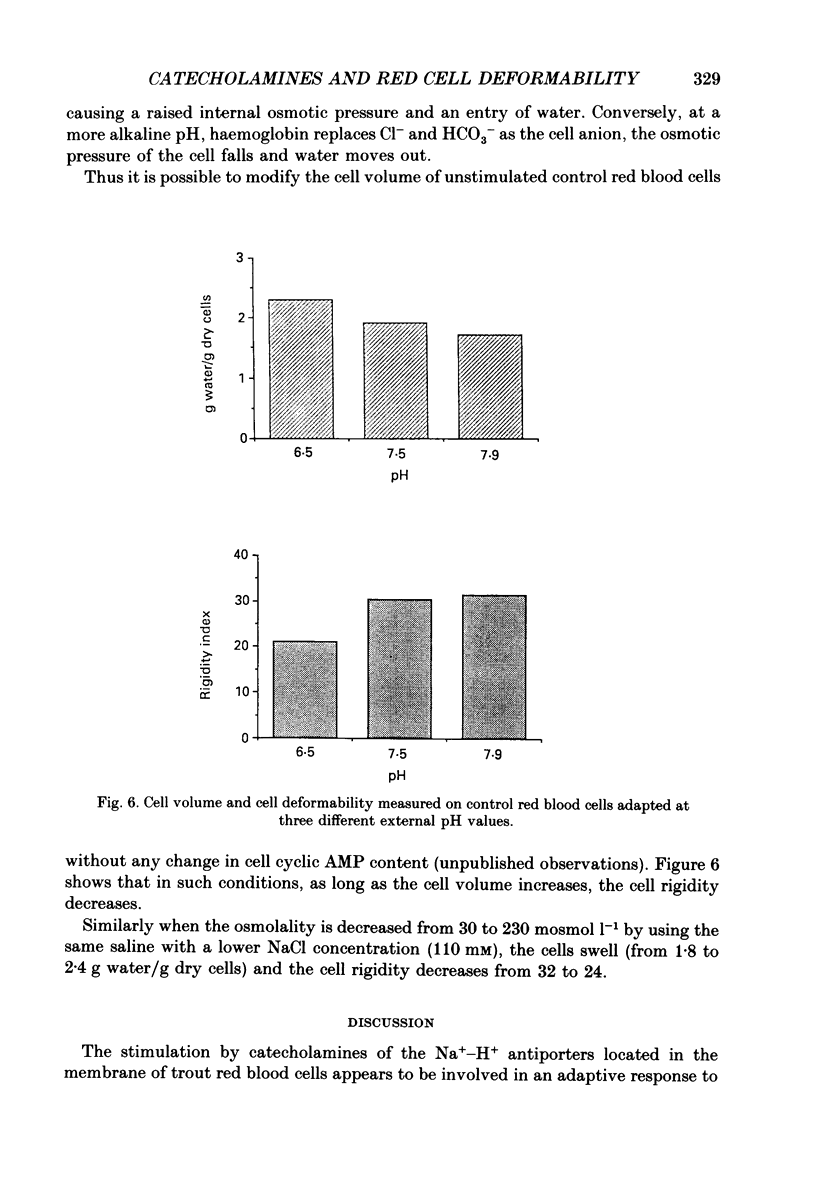



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman L. Protein cytoskeleton. Functional or futile phosphorus? Nature. 1988 Aug 25;334(6184):653–654. doi: 10.1038/334653a0. [DOI] [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. Hormone-induced co-transport with specific pharmacological properties in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 May;350:137–157. doi: 10.1113/jphysiol.1984.sp015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Catecholamine-induced transport systems in trout erythrocyte. Na+/H+ countertransport or NaCl cotransport? J Gen Physiol. 1986 Apr;87(4):551–566. doi: 10.1085/jgp.87.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Control of cell volume and ion transport by beta-adrenergic catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1987 Jan;382:123–144. doi: 10.1113/jphysiol.1987.sp016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Ion movements and volume changes induced by catecholamines in erythrocytes of rainbow trout: effect of pH. J Physiol. 1987 Jan;382:145–157. doi: 10.1113/jphysiol.1987.sp016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne P. K., Cossins A. R. On the instability of K+ influx in erythrocytes of the rainbow trout, Salmo gairdneri, and the role of catecholamine hormones in maintaining in vivo influx activity. J Exp Biol. 1982 Dec;101:93–104. doi: 10.1242/jeb.101.1.93. [DOI] [PubMed] [Google Scholar]
- Claireaux G., Thomas S., Fievet B., Motais R. Adaptive respiratory responses of trout to acute hypoxia. II. Blood oxygen carrying properties during hypoxia. Respir Physiol. 1988 Oct;74(1):91–98. doi: 10.1016/0034-5687(88)90143-0. [DOI] [PubMed] [Google Scholar]
- Fievet B., Claireaux G., Thomas S., Motais R. Adaptive respiratory responses of trout to acute hypoxia. III. Ion movements and pH changes in the red blood cell. Respir Physiol. 1988 Oct;74(1):99–113. doi: 10.1016/0034-5687(88)90144-2. [DOI] [PubMed] [Google Scholar]
- Fievet B., Motais R., Thomas S. Role of adrenergic-dependent H+ release from red cells in acidosis induced by hypoxia in trout. Am J Physiol. 1987 Feb;252(2 Pt 2):R269–R275. doi: 10.1152/ajpregu.1987.252.2.R269. [DOI] [PubMed] [Google Scholar]
- Hanss M. Erythrocyte filtrability measurement by the initial flow rate method. Biorheology. 1983;20(2):199–211. doi: 10.3233/bir-1983-20209. [DOI] [PubMed] [Google Scholar]
- Hughes G. M., Kikuchi Y. Effects of in vivo and in vitro changes in PO2 on the deformability of red blood cells of rainbow trout (Salmo gairdneri R.). J Exp Biol. 1984 Jul;111:253–257. doi: 10.1242/jeb.111.1.253. [DOI] [PubMed] [Google Scholar]
- Johnson R. M. pH effects on red cell deformability. Blood Cells. 1985;11(2):317-21, 323-4. [PubMed] [Google Scholar]
- Koutsouris D., Delatour-Hanss E., Hanss M. Physico-chemical factors of erythrocyte deformability. Biorheology. 1985;22(2):119–132. doi: 10.3233/bir-1985-22203. [DOI] [PubMed] [Google Scholar]
- Kury P. G., McConnell M. Regulation of Membrane Flexibility in Human Erythrocytes. Biochemistry. 1975 Jul;14(13):2798–2803. doi: 10.1021/bi00684a002. [DOI] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L. The measurement of erythrocyte deformability using micropore membranes. A sensitive technique with clinical applications. J Lab Clin Med. 1979 Jul;94(1):133–143. [PubMed] [Google Scholar]
- Mahé Y., Garcia-Romeu F., Motais R. Inhibition by amiloride of both adenylate cyclase activity and the Na+/H+ antiporter in fish erythrocytes. Eur J Pharmacol. 1985 Oct 22;116(3):199–206. doi: 10.1016/0014-2999(85)90154-2. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motais R., Garcia-Romeu F., Borgese F. The control of Na+/H+ exchange by molecular oxygen in trout erythrocytes. A possible role of hemoglobin as a transducer. J Gen Physiol. 1987 Aug;90(2):197–207. doi: 10.1085/jgp.90.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetens V., Lykkeboe G. Acute exposure of rainbow trout to mild and deep hypoxia: O2 affinity and O2 capacitance of arterial blood. Respir Physiol. 1985 Aug;61(2):221–235. doi: 10.1016/0034-5687(85)90128-8. [DOI] [PubMed] [Google Scholar]
- Thomas S., Fievet B., Claireaux G., Motais R. Adaptive respiratory responses of trout to acute hypoxia. I. Effects of water ionic composition on blood acid-base status response and gill morphology. Respir Physiol. 1988 Oct;74(1):77–89. doi: 10.1016/0034-5687(88)90142-9. [DOI] [PubMed] [Google Scholar]