Abstract
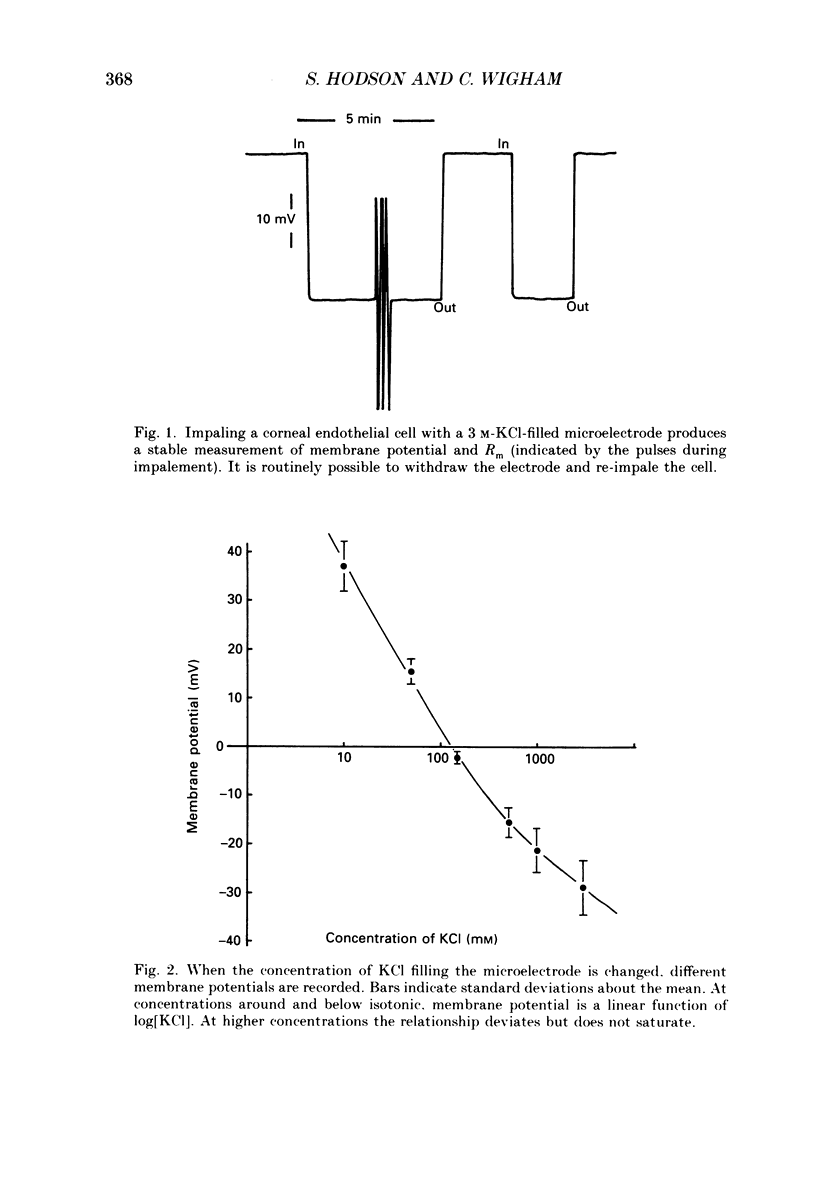
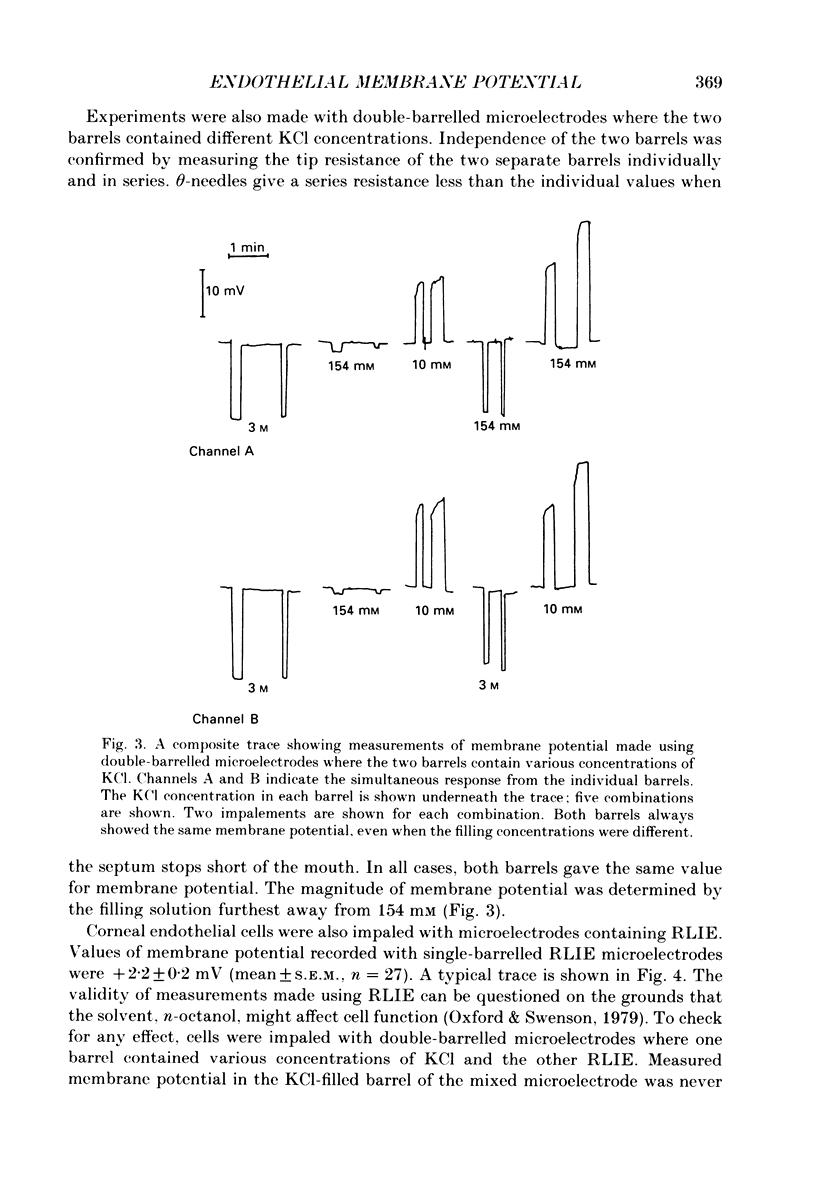
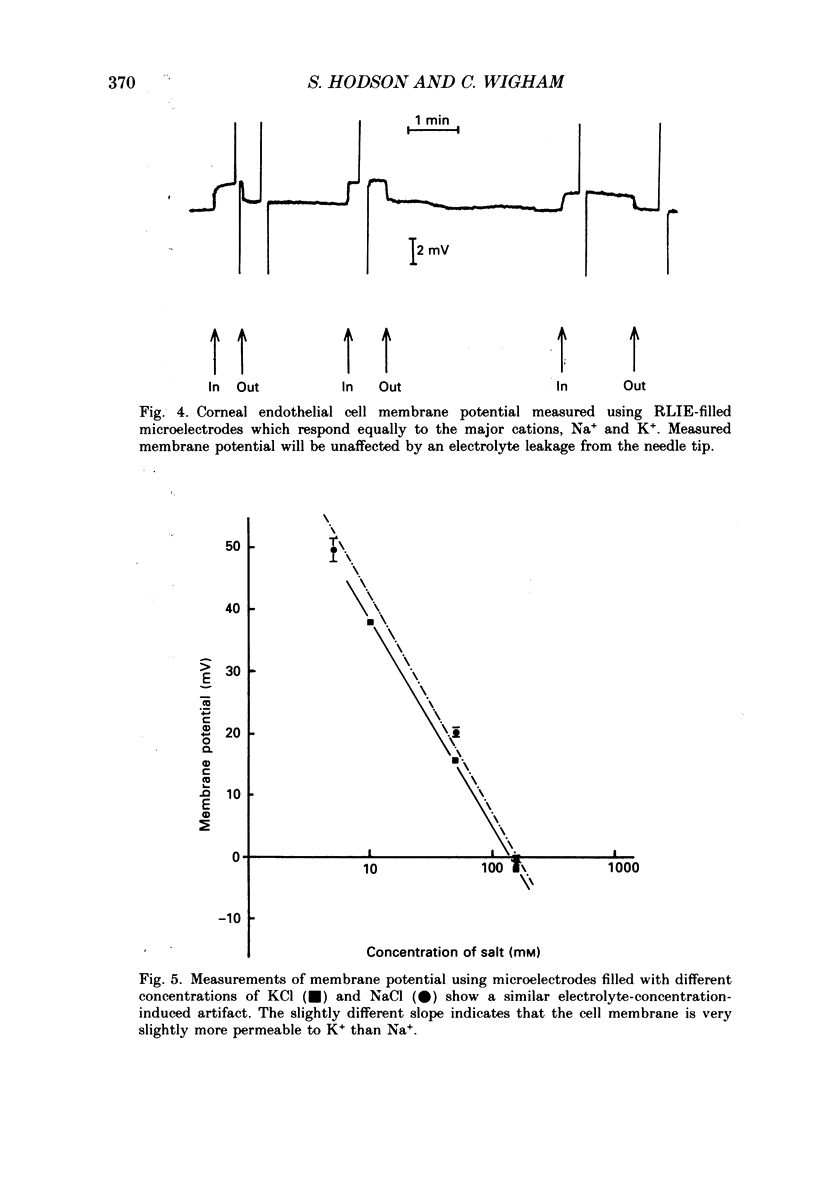
1. When rabbit corneal endothelial cells are impaled with 3 M-KCl-filled microelectrodes (Rt = 20-70 M omega) a stable membrane potential of -28.7 +/- 4.8 mV (mean +/- S.D., n = 400) is measured. 2. Varying the [KCl] of the filling solution causes a change in measured membrane potential; 154 mM gives typically -2 mV, 10 mM typically +37 mV. 3. Variation in membrane potential with different [KCl] cannot be ascribed to tip potential. Double-barrelled microelectrodes containing a different [KCl] in each barrel both give the same membrane potential when inserted into a cell. 4. Microelectrodes filled with a reference liquid ion exchanger (RLIE) give a membrane potential of +2 mV. 5. Impaling a cell with a double-barrelled microelectrode, one barrel containing KCl and the other RLIE, showed that the ion exchanger is not having a toxic effect on the cell and does not affect membrane potential measured by the KCl-filled barrel. 6. We suggest that microelectrodes containing non-isotonic concentrations of KCl generate a significant and artifactual change in membrane potential of corneal endothelial cells caused by the movement of excess KCl (originating from the microelectrode tip) across the plasma membrane where a liquid-junction potential is generated. 7. We further suggest that the physiological membrane potential of corneal endothelial cells is around zero. This could result from a solution of the constant field equation where: 0.9 PNa congruent to PK congruent to 3.2 PCl.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C. D., Lightfoot E. N., Schmidt F. P., Sy F. Diffusion effects of liquid-filled micropipettes: a pseudobinary analysis of electrolyte leakage. IEEE Trans Biomed Eng. 1972 Sep;19(5):372–375. doi: 10.1109/TBME.1972.324141. [DOI] [PubMed] [Google Scholar]
- Hodson S. A., Wigham C. G. Paracellular ionic and transcellular water diffusions across rabbit corneal endothelium. J Physiol. 1987 Apr;385:89–96. doi: 10.1113/jphysiol.1987.sp016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S., Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol. 1976 Dec;263(3):563–577. doi: 10.1113/jphysiol.1976.sp011645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S. Observations on the posterior membrane of the corneal endothelium. Exp Eye Res. 1969 Apr;8(2):99–101. doi: 10.1016/s0014-4835(69)80018-7. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Matthes H., Keller S. K., Wiederholt M. Anion dependence of electrical effects of bicarbonate and sodium on cultured bovine corneal endothelial cells. Pflugers Arch. 1985 Feb;403(2):175–185. doi: 10.1007/BF00584097. [DOI] [PubMed] [Google Scholar]
- Lim J. J., Fischbarg J. Intra-cellular potential of rabbit corneal endothelial cells. Exp Eye Res. 1979 Jun;28(6):619–626. doi: 10.1016/0014-4835(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Swenson R. P. n-Alkanols potentiate sodium channel inactivation in squid giant axons. Biophys J. 1979 Jun;26(3):585–590. doi: 10.1016/S0006-3495(79)85273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Cohen C. J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflugers Arch. 1981 Apr;390(1):96–98. doi: 10.1007/BF00582719. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Koch M. Intracellular potentials of isolated rabbit and human corneal endothelium. Exp Eye Res. 1978 Nov;27(5):511–518. doi: 10.1016/0014-4835(78)90136-7. [DOI] [PubMed] [Google Scholar]
- Wigham C., Hodson S. The movement of sodium across short-circuited rabbit corneal endothelium. Curr Eye Res. 1985 Dec;4(12):1241–1245. doi: 10.3109/02713688509017682. [DOI] [PubMed] [Google Scholar]


