Abstract
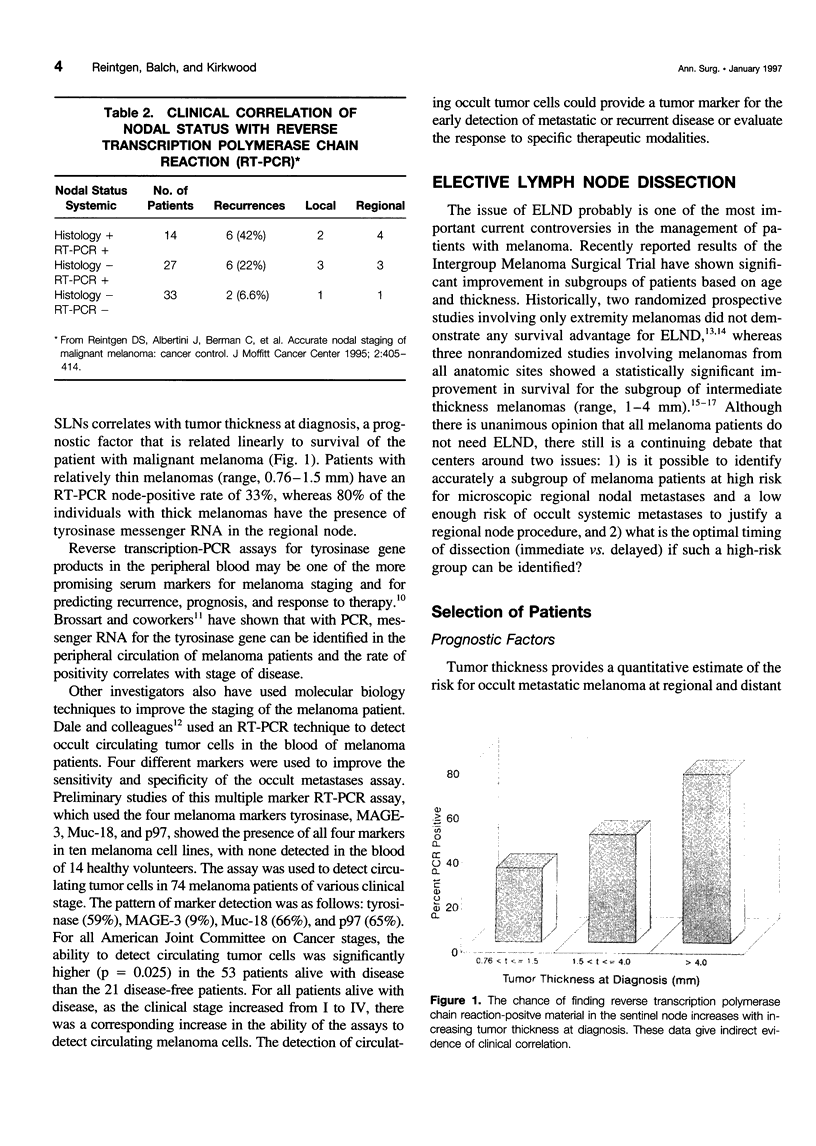
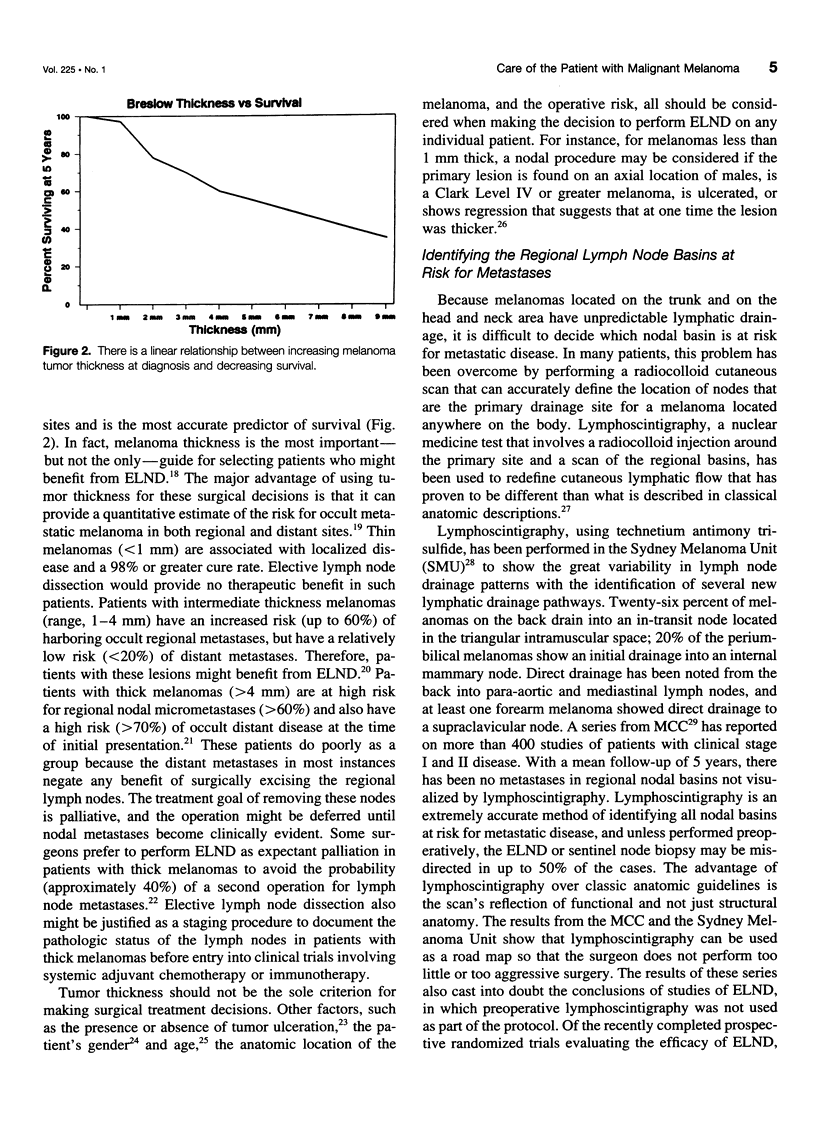
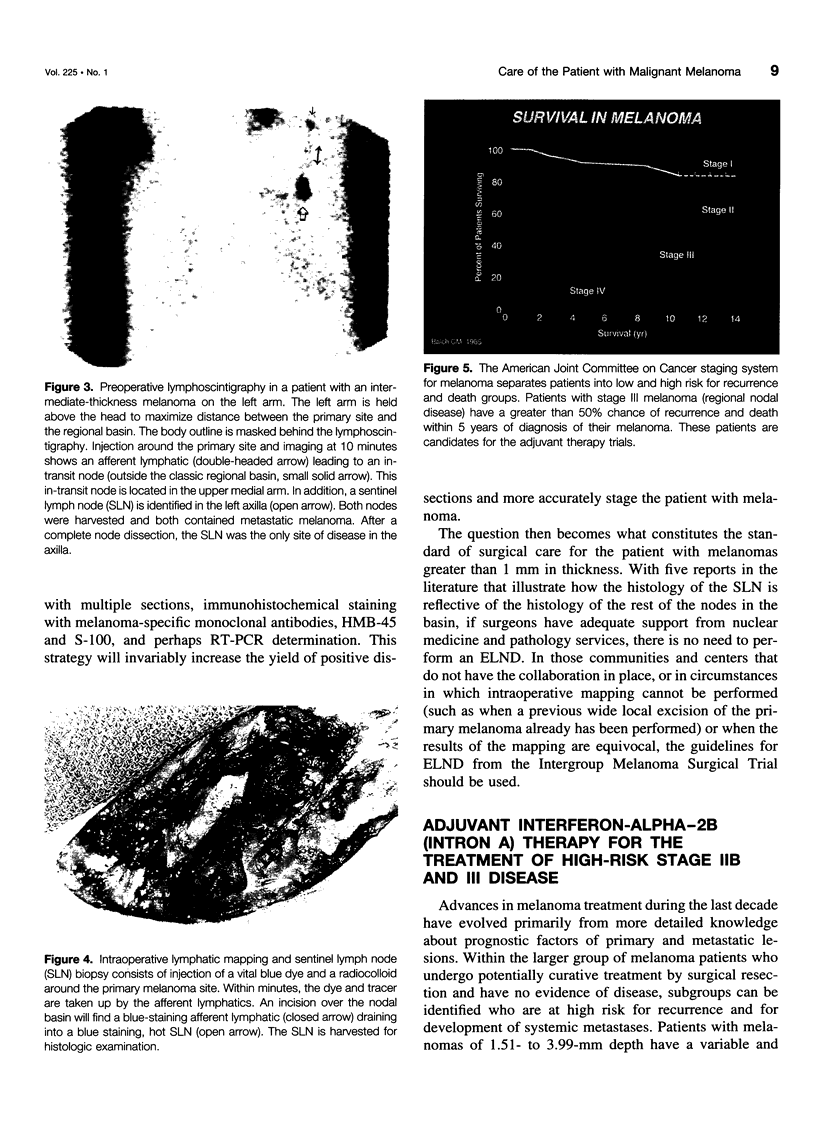
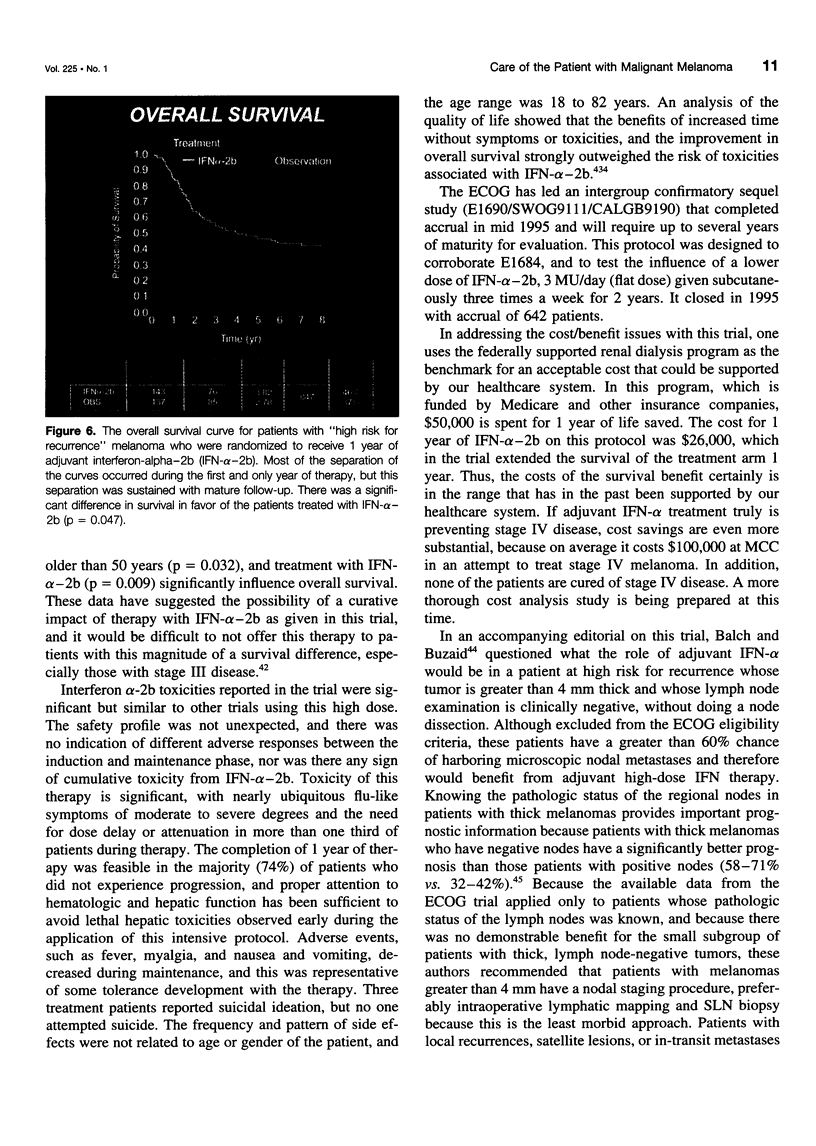
OBJECTIVE: The authors review the recent advances in the surgical care, staging, and adjuvant treatment of the patient with melanoma. SUMMARY BACKGROUND DATA: Melanoma care has not changed significantly in the last 20 years, and the controversy of elective lymph node dissections in this disease continues to be discussed. Two advances in the care of the patient with melanoma have occurred in the last 3 years to make this an exciting time for clinicians and to offer more hope for the patients with this disease. The concept of the sentinel lymph node (SLN), defined by Morton as the first node in the lymphatic basin that drains the primary melanoma, has been documented to contain the first site of metastatic disease. This technology can be used to stage nodally the melanoma patient, identifying the subgroup of patients (stage III) who have a 5-year survival rate less than 50%. Members of this group are candidates for effective adjuvant therapies. METHODS: A review of the surgical techniques of melanoma care, including recently reported new studies of elective node dissection (ELND) and SLN biopsy in patients with melanoma was performed. In addition, the Eastern Cooperative Oncology Group (ECOG) 1684 trial, which was the basis for the Food and Drug Administration approval of adjuvant interferon-alpha-2b (IFN-alpha-2b) is discussed. RESULTS: The Intergroup Melanoma Trial has reported a survival benefit for performing ELND in patients with melanoma and tumor thickness between 1 and 2 mm or in patients that are younger than 60 years of age. With six reports in the literature that show there is an order to melanoma nodal metastases and that the SLN histology is reflective of the histology of the remainder of the nodal basin, the more conservative SLN biopsy can be performed to adequately stage nodally the patient with melanoma. Patients with nodal metastases who are rendered free of disease with surgical resection have the most to benefit from adjuvant IF-alpha-2b. If one considers only the lymph node-positive group of patients, the survival benefit associate with adjuvant IFN is significant (p = 0.008). CONCLUSIONS: New standards of care for the melanoma patient have been established. Patients at high risk for recurrence have been shown to experience a survival benefit with adjuvant IFN-alpha-2b. With these data, the argument can be made that all patients with melanoma greater than 1 mm should have a nodal staging procedure. Selective lymphadenectomy with SLN biopsy is the least morbid procedure that can be used to obtain this information. If surgeons do not have the nuclear medicine or pathology support to perform lymphatic mapping, then the guidelines of the Intergroup Melanoma Study should be used to apply ELND in a selective fashion. In this way, patients are identified with micrometastatic disease early in their clinical course and can be offered the survival benefit of adjuvant therapy.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini J. J., Cruse C. W., Rapaport D., Wells K., Ross M., DeConti R., Berman C. G., Jared K., Messina J., Lyman G. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg. 1996 Feb;223(2):217–224. doi: 10.1097/00000658-199602000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P. F., Cruse C. W., Lyman G., Schroer K., Glass F., Reintgen D. S. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994 Nov;1(6):487–494. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Bartolucci A. A., Urist M. M., Karakousis C. P., Smith T. J., Temple W. J., Ross M. I., Jewell W. R., Mihm M. C. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg. 1996 Sep;224(3):255–266. doi: 10.1097/00000658-199609000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988 Jan;6(1):163–172. doi: 10.1200/JCO.1988.6.1.163. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Wilkerson J. A., Murad T. M., Soong S. J., Ingalls A. L., Maddox W. A. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980 Jun 15;45(12):3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossart P., Keilholz U., Willhauck M., Scheibenbogen C., Möhler T., Hunstein W. Hematogenous spread of malignant melanoma cells in different stages of disease. J Invest Dermatol. 1993 Dec;101(6):887–889. doi: 10.1111/1523-1747.ep12371713. [DOI] [PubMed] [Google Scholar]
- Brossart P., Schmier J. W., Krüger S., Willhauck M., Scheibenbogen C., Möhler T., Keilholz U. A polymerase chain reaction-based semiquantitative assessment of malignant melanoma cells in peripheral blood. Cancer Res. 1995 Sep 15;55(18):4065–4068. [PubMed] [Google Scholar]
- Coit D., Sauven P., Brennan M. Prognosis of thick cutaneous melanoma of the trunk and extremity. Arch Surg. 1990 Mar;125(3):322–326. doi: 10.1001/archsurg.1990.01410150044009. [DOI] [PubMed] [Google Scholar]
- Creagan E. T., Dalton R. J., Ahmann D. L., Jung S. H., Morton R. F., Langdon R. M., Jr, Kugler J., Rodrigue L. J. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol. 1995 Nov;13(11):2776–2783. doi: 10.1200/JCO.1995.13.11.2776. [DOI] [PubMed] [Google Scholar]
- Feinstein A. R., Sosin D. M., Wells C. K. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985 Jun 20;312(25):1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- Heller R., Becker J., Wasselle J., Baekey P., Cruse C. W., Cox C., King B., Wood H., Reintgen D. S. Detection of occult lymph node metastases in malignant melanoma. Ann Plast Surg. 1992 Jan;28(1):74–77. doi: 10.1097/00000637-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Heller R., Becker J., Wasselle J., Baekey P., Cruse W., Wells K., Cox C., King B., Reintgen D. S. Detection of submicroscopic lymph node metastases in patients with melanoma. Arch Surg. 1991 Dec;126(12):1455–1460. doi: 10.1001/archsurg.1991.01410360025005. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. M., Strawderman M. H., Ernstoff M. S., Smith T. J., Borden E. C., Blum R. H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996 Jan;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Krag D. N., Meijer S. J., Weaver D. L., Loggie B. W., Harlow S. P., Tanabe K. K., Laughlin E. H., Alex J. C. Minimal-access surgery for staging of malignant melanoma. Arch Surg. 1995 Jun;130(6):654–660. doi: 10.1001/archsurg.1995.01430060092018. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Kopecky K. J., Taylor C. W., Noyes R. D., Tuthill R. J., Hersh E. M., Feun L. G., Doroshow J. H., Flaherty L. E., Sondak V. K. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995 Nov 15;87(22):1710–1713. doi: 10.1093/jnci/87.22.1710. [DOI] [PubMed] [Google Scholar]
- Miliotes G., Albertini J., Berman C., Heller R., Messina J., Glass F., Cruse W., Rapaport D., Puleo C., Fenske N. The tumor biology of melanoma nodal metastases. Am Surg. 1996 Jan;62(1):81–88. [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., McCarthy W. H., Pearson L., Balch C. M., Soong S. J. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: results of surgical treatment in 1319 patients. Br J Surg. 1982 Feb;69(2):108–111. doi: 10.1002/bjs.1800690217. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Wen D. R., Wong J. H., Economou J. S., Cagle L. A., Storm F. K., Foshag L. J., Cochran A. J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- Norman J., Cruse C. W., Espinosa C., Cox C., Berman C., Clark R., Saba H., Wells K., Reintgen D. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991 Nov;162(5):432–437. doi: 10.1016/0002-9610(91)90255-c. [DOI] [PubMed] [Google Scholar]
- Norman J., Wells K., Kearney R., Cruse C. W., Berman C., Reintgen D. Identification of lymphatic drainage basins in patients with cutaneous melanoma. Semin Surg Oncol. 1993 May-Jun;9(3):224–227. [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1996. CA Cancer J Clin. 1996 Jan-Feb;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Reintgen D. S., Cox E. B., McCarty K. S., Jr, Vollmer R. T., Seigler H. F. Efficacy of elective lymph node dissection in patients with intermediate thickness primary melanoma. Ann Surg. 1983 Sep;198(3):379–385. doi: 10.1097/00000658-198309000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintgen D. S., Paull D. E., Seigler H. F., Cox E. B., McCarty K. S., Jr Sex related survival differences in instances of melanoma. Surg Gynecol Obstet. 1984 Oct;159(4):367–372. [PubMed] [Google Scholar]
- Reintgen D., Cruse C. W., Wells K., Berman C., Fenske N., Glass F., Schroer K., Heller R., Ross M., Lyman G. The orderly progression of melanoma nodal metastases. Ann Surg. 1994 Dec;220(6):759–767. doi: 10.1097/00000658-199412000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintgen D. Melanoma nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996 Jan;5(1):105–120. [PubMed] [Google Scholar]
- Reintgen D, Albertini J, Berman C, Cruse CW, Fenske N, Glass LF, Puleo C, Wang X, Wells K, Rapaport D. Accurate Nodal Staging of Malignant Melanoma. Cancer Control. 1995 Oct;2(5):405–414. doi: 10.1177/107327489500200504. [DOI] [PubMed] [Google Scholar]
- Reintgen D, Einstein AB., Jr The Role of Research in Cost Containment. Cancer Control. 1995 Oct;2(5):429–431. doi: 10.1177/107327489500200509. [DOI] [PubMed] [Google Scholar]
- Ross M. I., Reintgen D., Balch C. M. Selective lymphadenectomy: emerging role for lymphatic mapping and sentinel node biopsy in the management of early stage melanoma. Semin Surg Oncol. 1993 May-Jun;9(3):219–223. [PubMed] [Google Scholar]
- Schneebaum S., Briele H. A., Walker M. J., Greager J., Wood D. K., Ronan S. G., Patel M. K., Das Gupta T. K. Cutaneous thick melanoma. Prognosis and treatment. Arch Surg. 1987 Jun;122(6):707–711. doi: 10.1001/archsurg.1987.01400180089017. [DOI] [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Pritchard D. J., Soule E. H. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clin Proc. 1986 Sep;61(9):697–705. doi: 10.1016/s0025-6196(12)62768-2. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Vollmer R. T., Reintgen D. S., Seigler H. F. Lethal "thin" malignant melanoma. Identifying patients at risk. Ann Surg. 1988 Aug;208(2):150–161. doi: 10.1097/00000658-198808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Selby P., Southgate J., Pittman K., Bradley C., Blair G. E. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991 Nov 16;338(8777):1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorski V. V., Kirov S. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982 Jun 1;49(11):2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Rilke F., Luini A., Sacchini V., Galimberti V., Campa T., Dei Bei E., Greco M., Magni A., Merson M. Distribution of axillary node metastases by level of invasion. An analysis of 539 cases. Cancer. 1987 Feb 15;59(4):682–687. doi: 10.1002/1097-0142(19870215)59:4<682::aid-cncr2820590403>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wang X., Heller R., VanVoorhis N., Cruse C. W., Glass F., Fenske N., Berman C., Leo-Messina J., Rappaport D., Wells K. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann Surg. 1994 Dec;220(6):768–774. doi: 10.1097/00000658-199412000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]