Abstract
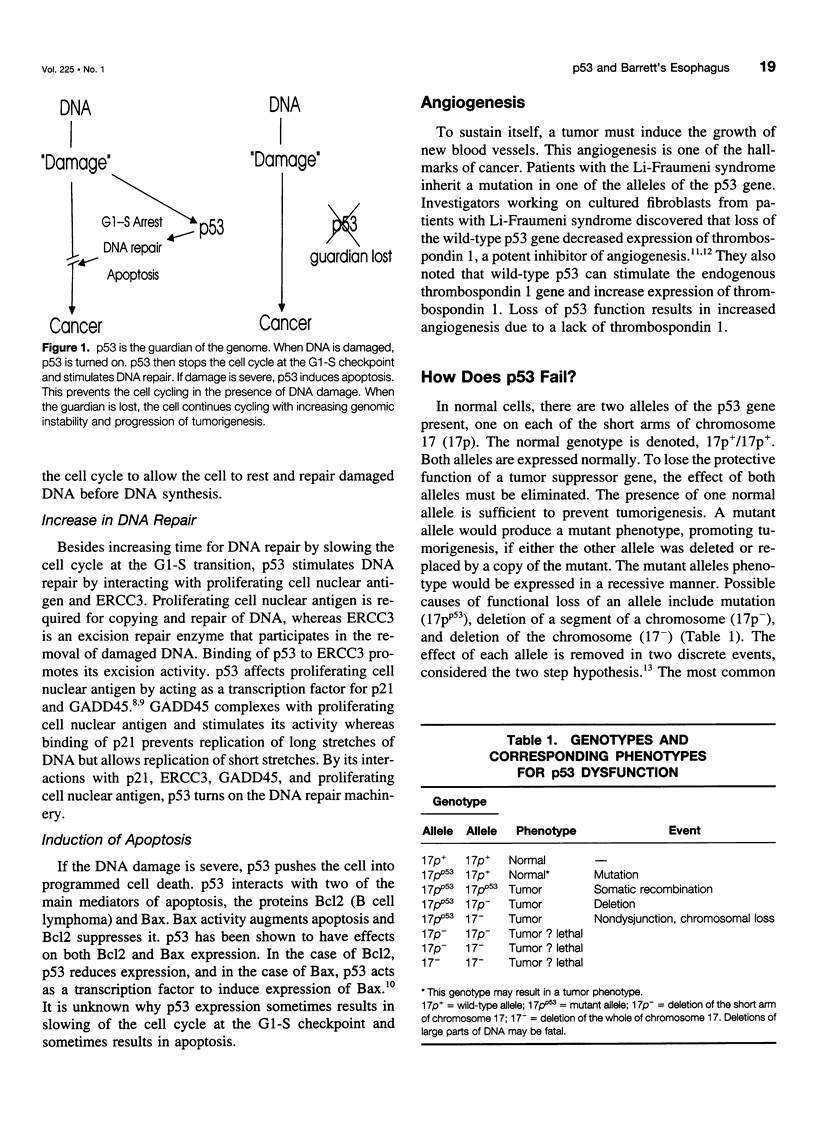
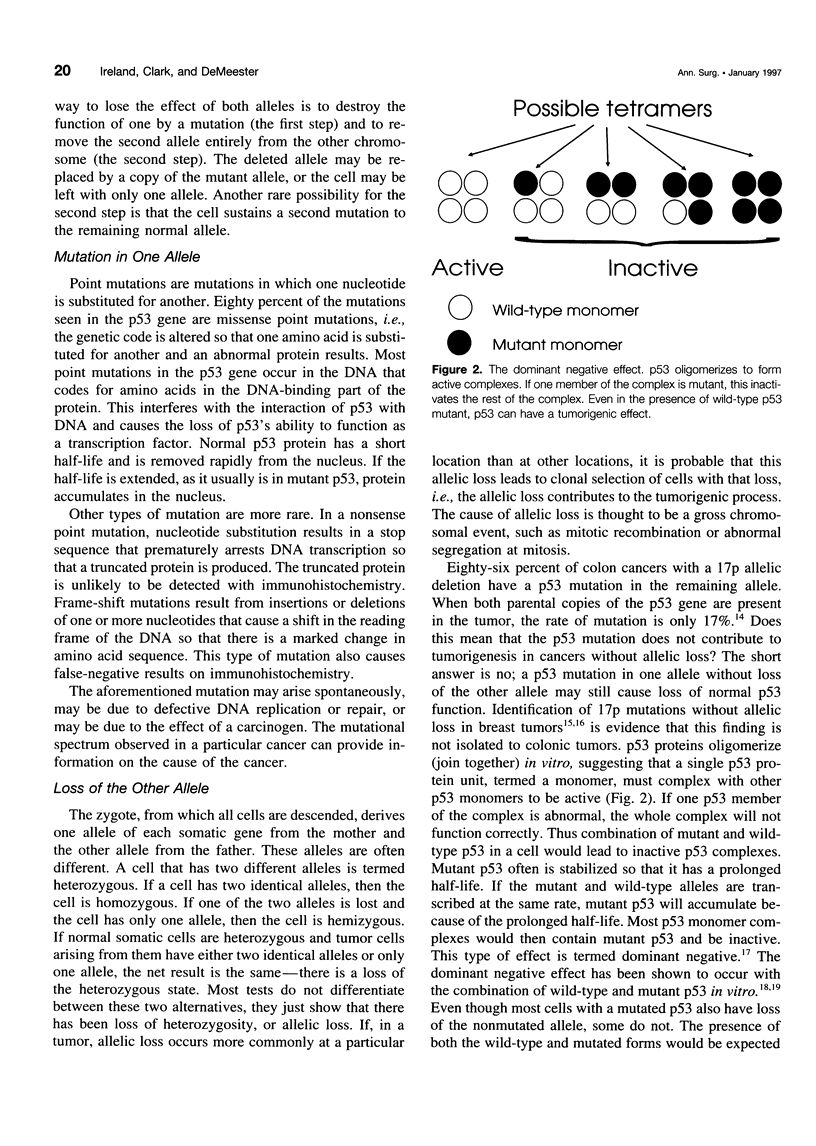
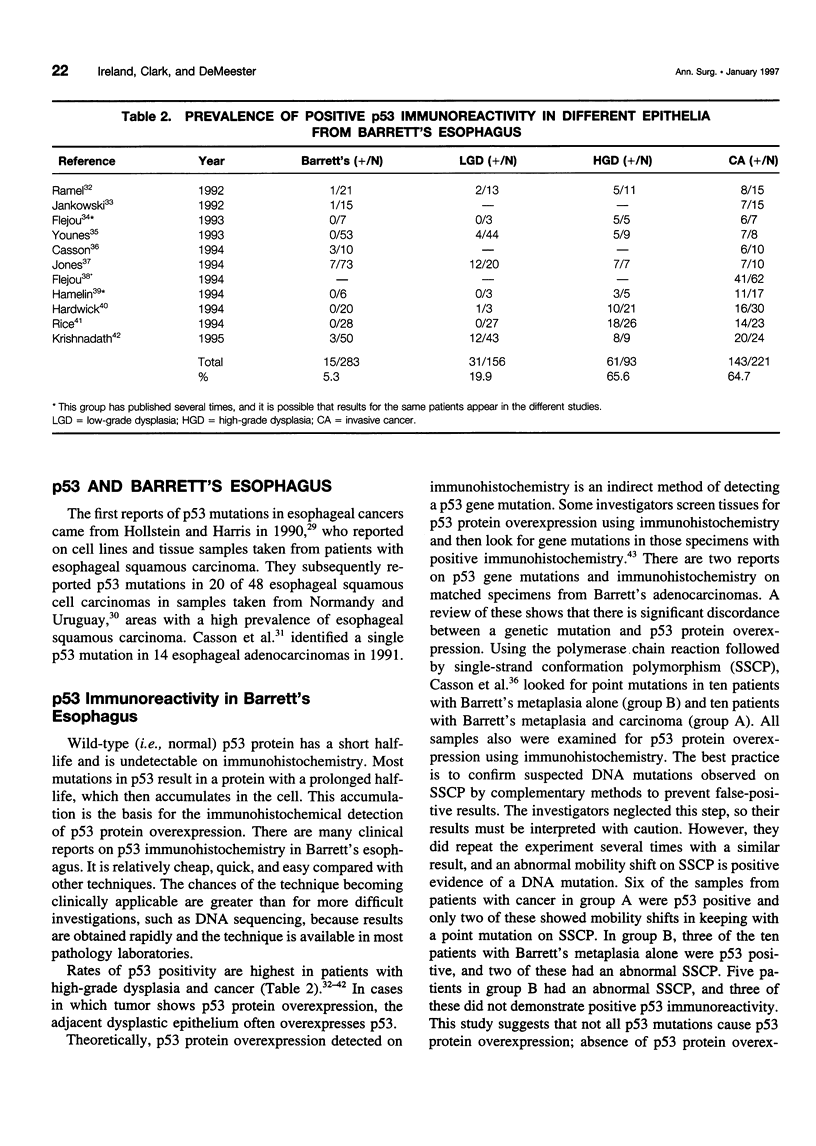
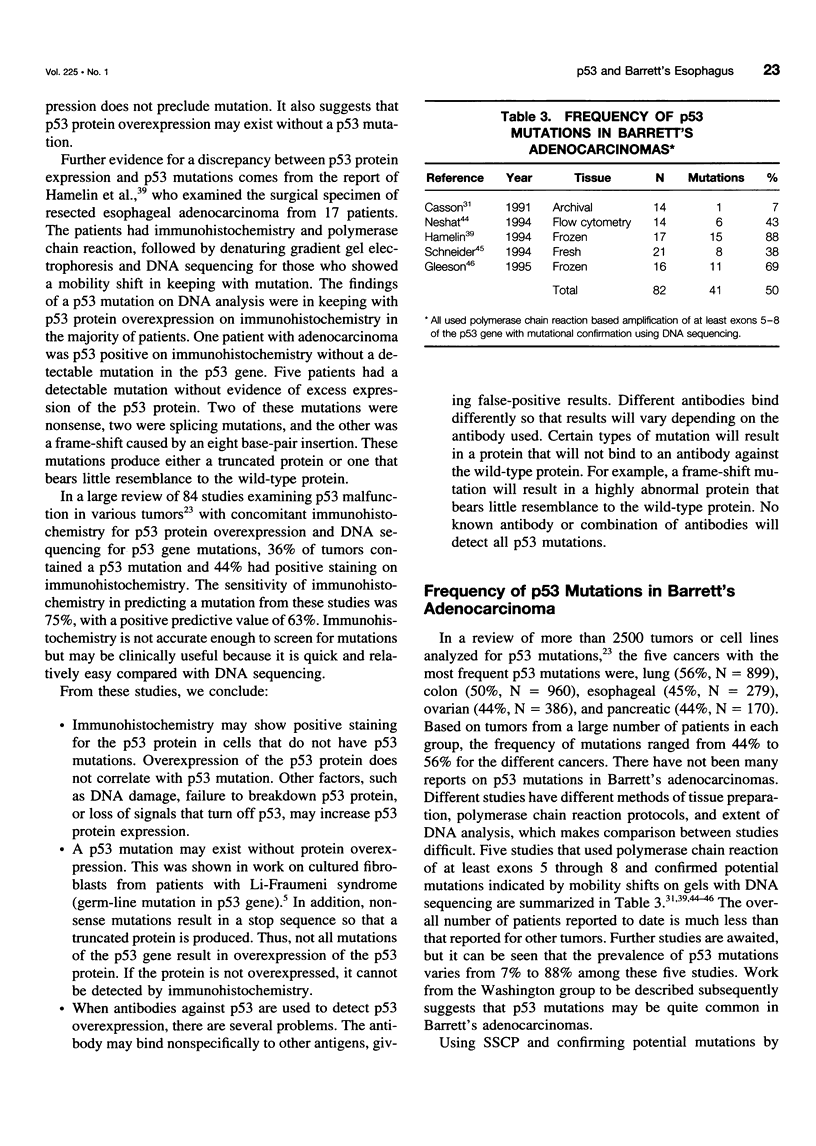
OBJECTIVE: The authors provide an updated review the molecular biology of the p53 tumor suppressor gene with reference to its role in the malignant degeneration of Barrett's esophagus. SUMMARY BACKGROUND DATA: Appreciation of the function of the tumor suppressor gene p53 has given new insight into regulation of the cell cycle, and the gene appears to play an important role in many solid tumors. Esophageal adenocarcinoma is increasing in frequency in the western world at an alarming rate and is unique because there is a clear metaplasia (Barrett's mucosa)/ dysplasia/carcinoma sequence. p53 malfunction arises as an early event in this carcinogenic process and has been demonstrated in patients with nondysplastic Barrett's metaplasia. The possible causes of p53 malfunction in this setting are discussed. The most reliable method for the detection of p53 mutations is DNA sequencing. p53 immunohistochemistry appears too insensitive to act as a reliable marker for the presence of a mutation and cannot be used as a reliable marker for the future development of cancer. CONCLUSIONS: High-grade dysplasia within Barrett's mucosa remains the best clinical predictor of adenocarcinoma. The mutational spectrum observed in these tumors should provide clues to their etiology.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altorki N. K., Sunagawa M., Little A. G., Skinner D. B. High-grade dysplasia in the columnar-lined esophagus. Am J Surg. 1991 Jan;161(1):97–100. doi: 10.1016/0002-9610(91)90367-m. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990 Aug 31;249(4972):970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Attwood S. E., Smyrk T. C., DeMeester T. R., Mirvish S. S., Stein H. J., Hinder R. A. Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery. 1992 May;111(5):503–510. [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Barnes D. M., Dublin E. A., Fisher C. J., Levison D. A., Millis R. R. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol. 1993 May;24(5):469–476. doi: 10.1016/0046-8177(93)90158-d. [DOI] [PubMed] [Google Scholar]
- Bergh J., Norberg T., Sjögren S., Lindgren A., Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med. 1995 Oct;1(10):1029–1034. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- Blot W. J., Devesa S. S., Fraumeni J. F., Jr Continuing climb in rates of esophageal adenocarcinoma: an update. JAMA. 1993 Sep 15;270(11):1320–1320. [PubMed] [Google Scholar]
- Blot W. J., Devesa S. S., Kneller R. W., Fraumeni J. F., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991 Mar 13;265(10):1287–1289. [PubMed] [Google Scholar]
- Blount P. L., Galipeau P. C., Sanchez C. A., Neshat K., Levine D. S., Yin J., Suzuki H., Abraham J. M., Meltzer S. J., Reid B. J. 17p allelic losses in diploid cells of patients with Barrett's esophagus who develop aneuploidy. Cancer Res. 1994 May 1;54(9):2292–2295. [PubMed] [Google Scholar]
- Blount P. L., Meltzer S. J., Yin J., Huang Y., Krasna M. J., Reid B. J. Clonal ordering of 17p and 5q allelic losses in Barrett dysplasia and adenocarcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3221–3225. doi: 10.1073/pnas.90.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M. T., Lawlor P., Byrne P. J., Walsh T. N., Hennessy T. P. Ambulatory oesophageal bile reflux monitoring in Barrett's oesophagus. Br J Surg. 1995 May;82(5):657–660. doi: 10.1002/bjs.1800820528. [DOI] [PubMed] [Google Scholar]
- Cameron A. J. Barrett's esophagus and adenocarcinoma: from the family to the gene. Gastroenterology. 1992 Apr;102(4 Pt 1):1421–1424. doi: 10.1016/0016-5085(92)90789-2. [DOI] [PubMed] [Google Scholar]
- Casson A. G., Manolopoulos B., Troster M., Kerkvliet N., O'Malley F., Inculet R., Finley R., Roth J. A. Clinical implications of p53 gene mutation in the progression of Barrett's epithelium to invasive esophageal cancer. Am J Surg. 1994 Jan;167(1):52–57. doi: 10.1016/0002-9610(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Casson A. G., Mukhopadhyay T., Cleary K. R., Ro J. Y., Levin B., Roth J. A. p53 gene mutations in Barrett's epithelium and esophageal cancer. Cancer Res. 1991 Aug 15;51(16):4495–4499. [PubMed] [Google Scholar]
- Clark G. W., Smyrk T. C., Mirvish S. S., Anselmino M., Yamashita Y., Hinder R. A., DeMeester T. R., Birt D. F. Effect of gastroduodenal juice and dietary fat on the development of Barrett's esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994 May;1(3):252–261. doi: 10.1007/BF02303531. [DOI] [PubMed] [Google Scholar]
- Culotta E., Koshland D. E., Jr p53 sweeps through cancer research. Science. 1993 Dec 24;262(5142):1958–1961. doi: 10.1126/science.7903477. [DOI] [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- Davidoff A. M., Humphrey P. A., Iglehart J. D., Marks J. R. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5006–5010. doi: 10.1073/pnas.88.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhaylongsod F. G., Gottfried M. R., Iglehart J. D., Vaughn A. L., Wolfe W. G. The significance of c-erb B-2 and p53 immunoreactivity in patients with adenocarcinoma of the esophagus. Ann Surg. 1995 Jun;221(6):677–684. doi: 10.1097/00000658-199506000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrig D., Elmajian D., Groshen S., Freeman J. A., Stein J. P., Chen S. C., Nichols P. W., Skinner D. G., Jones P. A., Cote R. J. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994 Nov 10;331(19):1259–1264. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- Fléjou J. F., Paraf F., Potet F., Muzeau F., Fékété F., Hénin D. p53 protein expression in Barrett's adenocarcinoma: a frequent event with no prognostic significance. Histopathology. 1994 May;24(5):487–489. doi: 10.1111/j.1365-2559.1994.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Fléjou J. F., Potet F., Muzeau F., Le Pelletier F., Fékété F., Hénin D. Overexpression of p53 protein in Barrett's syndrome with malignant transformation. J Clin Pathol. 1993 Apr;46(4):330–333. doi: 10.1136/jcp.46.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Gleeson C. M., Sloan J. M., McGuigan J. A., Ritchie A. J., Russell S. E. Base transitions at CpG dinucleotides in the p53 gene are common in esophageal adenocarcinoma. Cancer Res. 1995 Aug 1;55(15):3406–3411. [PubMed] [Google Scholar]
- Gray M. R., Donnelly R. J., Kingsnorth A. N. The role of smoking and alcohol in metaplasia and cancer risk in Barrett's columnar lined oesophagus. Gut. 1993 Jun;34(6):727–731. doi: 10.1136/gut.34.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Hamelin R., Fléjou J. F., Muzeau F., Potet F., Laurent-Puig P., Fékété F., Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994 Oct;107(4):1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Hardwick R. H., Shepherd N. A., Moorghen M., Newcomb P. V., Alderson D. Adenocarcinoma arising in Barrett's oesophagus: evidence for the participation of p53 dysfunction in the dysplasia/carcinoma sequence. Gut. 1994 Jun;35(6):764–768. doi: 10.1136/gut.35.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Harris C. C. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993 Dec 24;262(5142):1980–1981. doi: 10.1126/science.8266092. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M. C., Metcalf R. A., Welsh J. A., Montesano R., Harris C. C. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9958–9961. doi: 10.1073/pnas.87.24.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M. C., Peri L., Mandard A. M., Welsh J. A., Montesano R., Metcalf R. A., Bak M., Harris C. C. Genetic analysis of human esophageal tumors from two high incidence geographic areas: frequent p53 base substitutions and absence of ras mutations. Cancer Res. 1991 Aug 1;51(15):4102–4106. [PubMed] [Google Scholar]
- Huang Y., Meltzer S. J., Yin J., Tong Y., Chang E. H., Srivastava S., McDaniel T., Boynton R. F., Zou Z. Q. Altered messenger RNA and unique mutational profiles of p53 and Rb in human esophageal carcinomas. Cancer Res. 1993 Apr 15;53(8):1889–1894. [PubMed] [Google Scholar]
- Iftikhar S. Y., Ledingham S., Steele R. J., Evans D. F., Lendrum K., Atkinson M., Hardcastle J. D. Bile reflux in columnar-lined Barrett's oesophagus. Ann R Coll Surg Engl. 1993 Nov;75(6):411–416. [PMC free article] [PubMed] [Google Scholar]
- Jankowski J., Coghill G., Hopwood D., Wormsley K. G. Oncogenes and onco-suppressor gene in adenocarcinoma of the oesophagus. Gut. 1992 Aug;33(8):1033–1038. doi: 10.1136/gut.33.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. R., Davidson A. G., Summers C. L., Murray G. F., Quinlan D. C. Potential application of p53 as an intermediate biomarker in Barrett's esophagus. Ann Thorac Surg. 1994 Mar;57(3):598–603. doi: 10.1016/0003-4975(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Buckley J. D., Henderson B. E., Ross R. K., Pike M. C. From gene to carcinogen: a rapidly evolving field in molecular epidemiology. Cancer Res. 1991 Jul 1;51(13):3617–3620. [PubMed] [Google Scholar]
- Kauer W. K., Burdiles P., Ireland A. P., Clark G. W., Peters J. H., Bremner C. G., DeMeester T. R. Does duodenal juice reflux into the esophagus of patients with complicated GERD? Evaluation of a fiberoptic sensor for bilirubin. Am J Surg. 1995 Jan;169(1):98–104. doi: 10.1016/s0002-9610(99)80116-0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Krishnadath K. K., Tilanus H. W., van Blankenstein M., Bosman F. T., Mulder A. H. Accumulation of p53 protein in normal, dysplastic, and neoplastic Barrett's oesophagus. J Pathol. 1995 Feb;175(2):175–180. doi: 10.1002/path.1711750204. [DOI] [PubMed] [Google Scholar]
- Latta R. K., Fiander H., Ross N. W., Simpson C., Schneider H. Toxicity of bile acids to colon cancer cell lines. Cancer Lett. 1993 Jul 16;70(3):167–173. doi: 10.1016/0304-3835(93)90227-z. [DOI] [PubMed] [Google Scholar]
- Lillemoe K. D., Gadacz T. R., Harmon J. W. Bile absorption occurs during disruption of the esophageal mucosal barrier. J Surg Res. 1983 Jul;35(1):57–62. doi: 10.1016/0022-4804(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Lozano J. C., Nakazawa H., Cros M. P., Cabral R., Yamasaki H. G-->A mutations in p53 and Ha-ras genes in esophageal papillomas induced by N-nitrosomethylbenzylamine in two strains of rats. Mol Carcinog. 1994 Jan;9(1):33–39. doi: 10.1002/mc.2940090107. [DOI] [PubMed] [Google Scholar]
- Magewu A. N., Jones P. A. Ubiquitous and tenacious methylation of the CpG site in codon 248 of the p53 gene may explain its frequent appearance as a mutational hot spot in human cancer. Mol Cell Biol. 1994 Jun;14(6):4225–4232. doi: 10.1128/mcb.14.6.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Davidoff A. M., Kerns B. J., Humphrey P. A., Pence J. C., Dodge R. K., Clarke-Pearson D. L., Iglehart J. D., Bast R. C., Jr, Berchuck A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991 Jun 1;51(11):2979–2984. [PubMed] [Google Scholar]
- Marx J. New link found between p53 and DNA repair. Science. 1994 Nov 25;266(5189):1321–1322. doi: 10.1126/science.7973719. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A., Cook A. C. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991 Jan;11(1):12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991 May 31;65(5):765–774. doi: 10.1016/0092-8674(91)90384-b. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995 Jun 29;93(1):17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Moore J. H., Lesser E. J., Erdody D. H., Natale R. B., Orringer M. B., Beer D. G. Intestinal differentiation and p53 gene alterations in Barrett's esophagus and esophageal adenocarcinoma. Int J Cancer. 1994 Feb 15;56(4):487–493. doi: 10.1002/ijc.2910560406. [DOI] [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Blount P. L., Levine D. S., Joslyn G., Reid B. J. p53 mutations in Barrett's adenocarcinoma and high-grade dysplasia. Gastroenterology. 1994 Jun;106(6):1589–1595. doi: 10.1016/0016-5085(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Ohgaki H., Hard G. C., Hirota N., Maekawa A., Takahashi M., Kleihues P. Selective mutation of codons 204 and 213 of the p53 gene in rat tumors induced by alkylating N-nitroso compounds. Cancer Res. 1992 May 15;52(10):2995–2998. [PubMed] [Google Scholar]
- Palley S. L., Sampliner R. E., Garewal H. S. Management of high-grade dysplasia in Barrett's esophagus. J Clin Gastroenterol. 1989 Aug;11(4):369–372. [PubMed] [Google Scholar]
- Pera M., Cameron A. J., Trastek V. F., Carpenter H. A., Zinsmeister A. R. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993 Feb;104(2):510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- Pera M., Cardesa A., Bombi J. A., Ernst H., Pera C., Mohr U. Influence of esophagojejunostomy on the induction of adenocarcinoma of the distal esophagus in Sprague-Dawley rats by subcutaneous injection of 2,6-dimethylnitrosomorpholine. Cancer Res. 1989 Dec 1;49(23):6803–6808. [PubMed] [Google Scholar]
- Pera M., Trastek V. F., Carpenter H. A., Fernandez P. L., Cardesa A., Mohr U., Pairolero P. C. Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg. 1993 Jun;55(6):1386–1393. doi: 10.1016/0003-4975(93)91077-z. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Clark G. W., Ireland A. P., Chandrasoma P., Smyrk T. C., DeMeester T. R. Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg. 1994 Nov;108(5):813–822. [PubMed] [Google Scholar]
- Ramel S., Reid B. J., Sanchez C. A., Blount P. L., Levine D. S., Neshat K., Haggitt R. C., Dean P. J., Thor K., Rabinovitch P. S. Evaluation of p53 protein expression in Barrett's esophagus by two-parameter flow cytometry. Gastroenterology. 1992 Apr;102(4 Pt 1):1220–1228. [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Rabinovitch P. S. Barrett's esophagus. Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987 Jul;93(1):1–11. [PubMed] [Google Scholar]
- Rice T. W., Goldblum J. R., Falk G. W., Tubbs R. R., Kirby T. J., Casey G. p53 immunoreactivity in Barrett's metaplasia, dysplasia, and carcinoma. J Thorac Cardiovasc Surg. 1994 Dec;108(6):1132–1137. [PubMed] [Google Scholar]
- Schmutte C., Rideout W. M., 3rd, Shen J. C., Jones P. A. Mutagenicity of nitric oxide is not caused by deamination of cytosine or 5-methylcytosine in double-stranded DNA. Carcinogenesis. 1994 Dec;15(12):2899–2903. doi: 10.1093/carcin/15.12.2899. [DOI] [PubMed] [Google Scholar]
- Schmutte C., Yang A. S., Beart R. W., Jones P. A. Base excision repair of U:G mismatches at a mutational hotspot in the p53 gene is more efficient than base excision repair of T:G mismatches in extracts of human colon tumors. Cancer Res. 1995 Sep 1;55(17):3742–3746. [PubMed] [Google Scholar]
- Schweitzer E. J., Harmon J. W., Bass B. L., Batzri S. Bile acid efflux precedes mucosal barrier disruption in the rabbit esophagus. Am J Physiol. 1984 Nov;247(5 Pt 1):G480–G485. doi: 10.1152/ajpgi.1984.247.5.G480. [DOI] [PubMed] [Google Scholar]
- Scott N., Sagar P., Stewart J., Blair G. E., Dixon M. F., Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991 Feb;63(2):317–319. doi: 10.1038/bjc.1991.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. C., Rideout W. M., 3rd, Jones P. A. The rate of hydrolytic deamination of 5-methylcytosine in double-stranded DNA. Nucleic Acids Res. 1994 Mar 25;22(6):972–976. doi: 10.1093/nar/22.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. J., Andrews A. W. Bile acids: co-mutagenic activity in the Salmonella-mammalian-microsome mutagenicity test: brief communication. J Natl Cancer Inst. 1977 Nov;59(5):1557–1559. doi: 10.1093/jnci/59.5.1557. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., Bae I., Chen C. Y., Gilmer T. M., Kastan M. B., O'Connor P. M., Fornace A. J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Stein H. J., Feussner H., Kauer W., DeMeester T. R., Siewert J. R. Alkaline gastroesophageal reflux: assessment by ambulatory esophageal aspiration and pH monitoring. Am J Surg. 1994 Jan;167(1):163–168. doi: 10.1016/0002-9610(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Streitz J. M., Jr, Andrews C. W., Jr, Ellis F. H., Jr Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg. 1993 Mar;105(3):383–388. [PubMed] [Google Scholar]
- Vaezi M. F., Richter J. E. Synergism of acid and duodenogastroesophageal reflux in complicated Barrett's esophagus. Surgery. 1995 Jun;117(6):699–704. doi: 10.1016/s0039-6060(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Brammar W. J., Lane D. P., Swallow J. E., Dolan C., Walker R. A. Loss of chromosome 17p13 sequences and mutation of p53 in human breast carcinomas. Oncogene. 1991 Mar;6(3):413–421. [PubMed] [Google Scholar]
- Vijeyasingam R., Darnton S. J., Jenner K., Allen C. A., Billingham C., Matthews H. R. Expression of p53 protein in oesophageal carcinoma: clinicopathological correlation and prognostic significance. Br J Surg. 1994 Nov;81(11):1623–1626. doi: 10.1002/bjs.1800811120. [DOI] [PubMed] [Google Scholar]
- Wang Y., You M., Reynolds S. H., Stoner G. D., Anderson M. W. Mutational activation of the cellular Harvey ras oncogene in rat esophageal papillomas induced by methylbenzylnitrosamine. Cancer Res. 1990 Mar 1;50(5):1591–1595. [PubMed] [Google Scholar]
- Younes M., Lebovitz R. M., Lechago L. V., Lechago J. p53 protein accumulation in Barrett's metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993 Dec;105(6):1637–1642. doi: 10.1016/0016-5085(93)91058-p. [DOI] [PubMed] [Google Scholar]


