RESUME
Contexte: Bien que les exercices aérobiques aient démontré leur efficacité pour ralentir le déclin cognitif et améliorer les symptômes psychologiques associés aux déficits cognitifs, ils peuvent ne pas être réalisables en raison de multiples handicaps. D'autres exercices doux avec des approches conscientes, tels que le "Yoga-like", ont été explorés mais manquent de preuves claires. Objectif: Évaluer l'efficacité d'une intervention "Yoga-like" sur les caractéristiques cognitives et psychologiques chez des patients atteints de la maladie d'Alzheimer (MA) légère à modérée. Méthodes: Nous proposons un essai contrôlé randomisé. Les patients atteints de MA légère à modérée capables de subir une évaluation neurocognitive et ne présentant pas de conditions contre-indiquant la respiration profonde ou les postures extrêmes seront assignés aléatoirement à un groupe d'intervention (GI: "Yoga-like") ou à un groupe témoin (GT: pas d'intervention). L'intervention "Yoga-like" consiste en 30 minutes d'exercices combinant respiration, postures, concentration et méditation, effectués trois fois par semaine pendant huit semaines. Les deux groupes subiront des tests neuropsychologiques au départ et après huit semaines, y compris l'attention, la résolution de problèmes, les capacités visuospatiales, l'humeur et les symptômes neuropsychiatriques. Résultats attendus: Il est prévu que les composantes de respiration, de concentration et de méditation de l'intervention améliorent l'attention, les capacités de résolution de problèmes et les symptômes comportementaux. Les composantes posturales devraient améliorer le contrôle visuospatial et l'équilibre. Enregistrement de l'essai: PACTR202407721329710 (https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=30602)
ABSTRACT
Background: While aerobic exercises have demonstrated efficacy in slowing cognitive decline and improving psychological symptoms associated with cognitive impairments, they may not be feasible due to multiple disabilities. Other gentle exercises with mindful approaches, such as “Yoga like”, have been explored but lack clear evidence. Aim: To assess the efficacy of a "Yoga-like" intervention on cognitive and psychological features in patients with mild to moderate Alzheimer Disease (AD). Methods: We propose a randomized controlled trial design. Patients with mild to moderate AD who are able to undergo neurocognitive assessment and do not have conditions contraindicating deep breathing or extreme postures will be randomly assigned to an intervention group (IG: Yoga like) or a control group (CG: no intervention). The ‘Yoga-like’ intervention consists of 30 minutes of exercises combining breathing, postures, concentration, and meditation, conducted three times a week over eight weeks. Both groups will undergo neuropsychological tests at baseline and after eight weeks, including attention, problem-solving, visuospatial abilities, mood and neuropsychiatric symptoms. Expected results: It is anticipated that the breathing, concentration, and meditation components of the intervention will improve attention, problem-solving abilities and behavioral symptoms. The postural components are expected to enhance visuospatial control and balance. Trial registration: PACTR202407721329710 (https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=30602)
Introduction
Increased life expectancy has led to a rise in cognitive impairments (CI), with Alzheimer's disease (AD) being the most prevalent cause (1, 2).
Initially, the disease was diagnosed based on the impairment of episodic memory but research has shown that numerous symptoms precede memory loss, including attention deficits, executive dysfunction, processing speed decline, language impairments, and visuospatial skills (2).
Additional symptoms are associated with the disease including anxiety, loss of self-image, and aggression 3.
CI in the elderly were related to many risk factors including educational level, occupational and social activities, sedentary lifestyle, cardiovascular risk factors, depression and genetic predisposition 4, 5.
The current (ie; year 2024) pharmacological treatment of AD focuses on symptomatic management 6, 7 .
While these medications can provide some relief from symptoms, they do not slow or prevent the progression of AD 8, are expensive and may have side effects 6-9.
Non-pharmacological interventions were based first on occupational therapy, but some researchers reported a positive correlation between increased physical activity and improve symptoms in patients with AD (10, 11).
Those exercises are more efficient when having multisensory stimulation (13-16).
Mind-body exercises, such as Yoga is one of the integrative exercises that has been introduced in the management of CI in the elderly 14 , 17-20 .
It is a multi-component mind-body practice, which combines physical postures, breathing exercises, and meditation 21.
Yoga can enhance the density of gray matter in areas of the brain that are linked to memory and emotional regulation (22-25).
However, existing studies, limited in patient numbers, do not provide sufficient evidence of Yoga efficacy in AD (14).
Additionally, the details of Yoga interventions are often inadequately reported (19, 20) and many Yoga interventions exclude the spiritual aspects (ie; meditation) of the practice that could have positive effects on concentration and memory (20).
Classical Yoga, originating thousands of years ago in India, includes eight pillars: physical postures (asanas), breathing exercises (pranayama), and meditation practice (19, 21, 25-27).
Those pillars could be a good solution for cognitive decline, anxiety and aggressively (27) but it may not be suitable for older adults with motor disorders and polyarthritis, conditions affecting 40% of the elderly in low- and middle-income countries (18).
“" Chair Yoga” (ie; “" Yoga-like”), a modified form designed for those unable to practice Yoga on the floor, is a safe and effective alternative for older adults with motor disorders and polyarthritis (28).
The aim of the present randomized clinical trial (RCT) protocol comprising two groups [Intervention group (IG): Modified Yoga, and control group (CG): no intervention] will be to examine the effects of a modified Yoga intervention program (ie; “" Yoga-like”) on neuropsychological assessment (ie; primary outcome), memory, executive function, visuospatial skills, problem-solving, and depression in patient with mild to moderate AD. The “" Yoga-like” program will be deemed clinically effective if it produces a change in neuropsychiatric inventory questionnaire (NPI-Q) exceeding the recommended minimal clinically important difference (MCID) of two (29, 30).
Methods
Study setting
This monocentric, prospective, RCT was designed in accordance with the guidelines of the SPIRIT (for standard protocol items: recommendations for interventional trials) 31.
Patients will be recruited during a 10-month period (ie; September 1, 2024, to June 1, 2025) from the Physical and Rehabilitation and Neurology departments(Sahloul Hospital, Sousse, Tunisia).
The study protocol received an ethical approval from the ethical committee of faculty of medicine of Sousse, Tunisia (Approval N° 244/ 2024).
Each patient’s tutor will be informed about the study aims and will receive a report of the patient’s explorations.
A written informed consent will be obtained from the patient’s tutor.
Participation in the study will be free of charge.
Data collection will be pseudonymised.
Collected data will be stored electronically on a passwordprotected computer.
The study was registered in the Pan African Clinical Trial Registry within the number PACTR202407721329710 (https://pactr.samrc.ac.za/ TrialDisplay.aspx?TrialID=30602).
Recruitment method, study population, and inclusion, non-inclusion, and exclusion criteria
Patients will be recruited by two ways.
First, from the patient database of the fore mentioned Neurology department, which includes patients/tutors' phone numbers and those of their tutors.
Second, from patients consulting the outpatient Neurology department, for cognitive disorder during the study period.
The information sheet of these patients includes systematically the telephone contact of the patients/tutors.
If a patient/ tutor cannot be reached after three phone calls over one week, the patient will not be included in the study.
Only patients diagnosed with mild to moderate stages of AD according to recommendations from the national institute on aging Alzheimer’s 32, having normal or corrected-to-normal vision and color perception, able to sit on a chair, and who don’t practice regular physical activity during the study period will be included in the protocol.
The following non-inclusion criteria will be applied: presence of cardiovascular or orthopedic conditions that could affect the ability to perform the proposed intervention tasks; heart or respiratory diseases that do not allow physical activity; severe cognitive disorders or severe psychiatric diseases; medications that affect posture or the ability to perform exercises.
Files of patients who do not complete the intervention (for the IG) or the final neuropsychological assessments(for both groups), or have missing data, will be excluded from the final analysis.
Sample size
The sample size was estimated using the formula 33 :
N = ((r+1) (Zα/2 + Z1-β) 2δ2)/MCID2, where
• “" N” is equal to n1 + n2(ie; sample sizes for the IG and CG);
• “" Zα/2” is the normal deviate for type I error(fixed at 5%,Zα/2 = 1.96);
• “" Z1-β” is the normal deviate for type II error (fixed at 80%, Z1-β= 0.84);
• “" r” (equal to n1/n2) is the ratio of the sample sizerequired for the two groups (here, r = 1 gives a sample size distribution of 1:1 for the IG and CG);
• “" δ” and “" MCID” are the pooled standard deviation(SD) and the MCID of the main outcome (ie; NPI-Q score).
Given the pioneering nature of this study at the time of its realization, “" δ” and “" MCID” values were fixed at two in NPI-Q score 30, 34.
Inserting the aforementioned data into the formula resulted in a sample size of 16 patients in each group.
Considering a potential power loss of 10% due to possible adverse effects, the revised sample size was calculated to be 20 patients in each group [18 = 16/(1-0.10)].
Study protocol and procedures, and randomization
The procedures will consist of four visits (V1 to V4) described in Box 1
Box 1. Schedule of enrolment, interventions, and assessments.
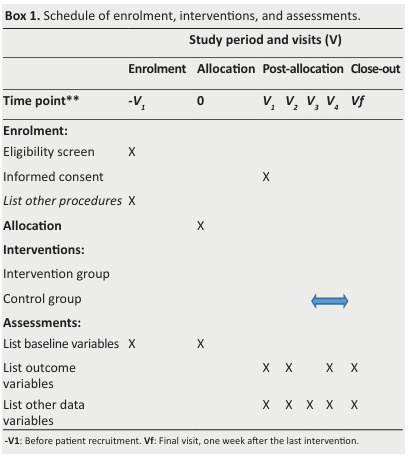
Visit 1 (V1)
All patients consulting for CI will undergo neuropsychological evaluation.
Those diagnosed with mild or moderate AD will undergo an interview (in the presence of their tutors) regarding the study, assessment,intervention, duration, and aims.
Once all inclusion and non-inclusion criteria will be met, the date for the assessment visit (V2) will be scheduled.
Any questions or concerns will be discussed by three physicians (RM,SN, and HH in the authors’ list).
Subsequently, both the physician and the patient's tutor will sign an informed consent form officially admitting the patient to the study.
A neurologist (SN in the authors’ list) will establish mild to moderate AD diagnosis during the expected period.
Visit 2 (V2)
DuringV2, patients will assess neurocognitive tests with a trained neurologist at baseline.
Visit 3 (V3)
During V3, patients will be randomly assigned to receive their first intervention, either IG or CG.
Randomization will be conducted by one investigator using a random list generated online through PubMed's random group generator (available at http://www.pubmed.de/tools/zufallsgenerator/?no_cache=1).
A randomization schema determined the assignment of patients to IG or CG.
A list was created with a chronological sequence to ensure clear assignment of each patient to one of the two groups.
The physicians responsible for randomization(HH in the authors’ list) entered each new patient's name, along with the time, date, and randomized number, into the patient's folder.
Due to economic considerations, the physicians were not blinded.
Once randomization is completed, the physician will schedule the first day of intervention.
Visit 4 (V4)
V4 will occur after the intervention, when patients will have final neurocognitive tests with one investigator (SN in the authors’ list).
Data collection
Data will be collected via a questionnaire consisting of two parts.
The total duration of the questionnaire is estimated to be 40 minutes (ie; ten minutes for the first part, 30 minutes for the second part).
If any patient could not complete the assessments due to attention problems, the neuropsychological tests will be divided in two visits(V2a and V2b) separated by a 24-36 hours.
Part 1: Socio-demographic and medical history
The following socio-demographic and medical history, which are identified as risk factors of CI (4, 5) will be evaluated: age (years), sex (male/female), origin (rural/ urban), family (live alone/ with family), profession and leisure activities (sports, gardening, dance), cardiovascular diseases (yes/no), history of depression (yes/no) and anxiety (yes/no), and family history of dementia (yes/no).
Part 2: Neurocognitive tests
MMSE Arabic version will be applied to all patients at baseline.
MMSE is a simple evaluation containing 11 questions that provides an overview of cognitive function with a maximum score of 30 ( 35, 36).
Scores between 26 and 30 indicate normal cognitive function, between 20 and 25 mild and 10 to 19 moderate cognitive disorder ( 36) (Box 2).
Box 2. Summary of different tests that will be used for patient’s neuropsychological assessment.
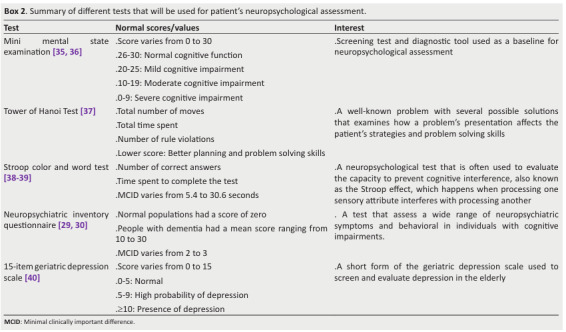
The following neurocognitive tests will be performed in this order (Box 2, Figure 1): Tower of Hanoi test 37, Stroop color and word test 38, 39, NPI-Q 29, 30, and 15 item of geriatric depression scale (GDS-15) 40.
Problem-solving skills will be assessed using the Tower of Hanoi test 37.
This task involves transferring disks among three pegs to recreate a pyramid pattern, considering the number of moves and time taken.
A five-disk variant will be used, and the test theoretically lasts 10 minutes 37.
Attention will be assessed using the Arabic version of the Stroop color and word test 38, 39.
Over five minutes, patients will respond to stimuli presented on a computer screen across four phases. They will identify shapes' colors, read color names in black ink, read color names in matching colors, and name the ink color of words printed in mismatched colors 39.
The MCID of Stroop test is 5.4 to 30.6 seconds 39.
This test lasts approximately five minutes.
NPI-Q [30] is a comprehensive tool used to assess a wide range of neuropsychiatric symptoms in individuals with dementia and other neurological conditions [29].
It evaluates behaviors and psychological symptoms including delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, and sleep and appetite disturbances.
The NPI-Q typically takes about five to 10 minutes to administer, involving detailed input from a caregiver or informant.
A change of approximately 2 to 3 points on the total NPI-Q score is often considered MCID, reflecting a meaningful shift in symptom severity 30.
Mood will be assessed using the GDS-15 [40] .
The latter is widely used in clinical and research settings among the geriatric population to screen for mood disorders and depression.
Scores of 0 to 5 are regarded as normal; those of 5 to 9 suggest a high probability of depression; and scores of 10 and more indicate the presence of depression.
This test last theoretically 10 minutes [40] .
Validity of Yoga concepts in health
The concepts of pranayama [21], Yama, and Niyama [41], Pratyahara [48], Dharana, Dhyana, and Samadhi originate from ancient yogic traditions and have been subjects of interest in various scientific studies [2], [21], [41]-[48].
Introduced in biomedical sciences and health, yoga has always been interpreted through its philosophy [42], but in the last decade (ie; 2014-2024), studies have shown the physiological effects of these concepts [43], [44].
Physiologic effects due to pranayama are characterized by decreased oxygen consumption, decreased heart rate, and decreased blood pressure, as well as increased theta wave amplitude in electroencephalography recordings, increased parasympathetic activity accompanied by feelings of alertness and reinvigoration 44.
Indeed, this diaphragmatic breathing stimulates the deep fascia of the abdomen and thorax, activates the vagus nerve and solar plexus, and modulates the autonomic nervous system, thereby reducing stress 44, 45.
The research specifically focusing on the clinical validity of Yama and Niyama is sparse.
However, these principles are often included as part of comprehensive yoga programs and many studies have proven effects of these concepts to reduce heart rate and improve autonomic regulation 43, 44.
Another pillar, Pratyahara 42, where patients are asked to withdraw their senses, seeks to calm the mind by lessening the influence of sensory stimulation 42.
Dharana, Dhyana, and Samadhi benefit the brain, as shown by electrophysiological and recent functional magnetic resonance imaging studies 43-45.
Overall, electroencephalographic measures indicate slowing subsequent to meditation, with theta and alpha activation related to proficiency of practice 43-45.
Sensory evoked potential assessment of concentrative meditation yields amplitude and latency changes for some components and practices 44.
Cognitive event-related potential evaluation of meditation implies that practice changes attentional allocation 44-46.
Neuroimaging studies indicate increased regional cerebral blood flow measures during meditation 43-45.
Another critical point in yoga practice in clinical trials is the reproducibility of these concepts and how to ensure the application of meditation.
However, some studies have identified how yoga teachers and classes can ensure fidelity to all pillars to achieve yoga benefits 47 , 48.
Figure 1. Illustration of the five applied psycho-cognitive tests.
Intervention
The intervention will include the eight pillars of Yoga applied while seated on a chair three times a week for eight weeks, 30 min per session 28.
The rehabilitation area is chosen to ensure concentration and meditation 28.
Chair height will be selected to ensure patient comfort and allow patient to rest their feet on the floor with a backrest that allows the patient to rest their back in an aligned position.
Sessions will be led by one therapist, a yoga teacher, with small group of five patients.
The session will begin with slow and deep breathing exercises 21, 28.
The therapist will educate patients on proper breathing techniques, known as Pranayama (Breath control) 21, 25-28.
Pranayama involves regulating the breath to enhance energy flow and mental clarity 21, 25 -28.
Patients will be instructed to place one hand on the chest and the other on the abdomen to feel abdominal expansion during inhalation and its contraction during slow exhalation 46.
Simultaneously with deep inhalation, patients will learn to contract their perineal muscles 49.
In addition to breathing techniques, the therapist will review two foundational pillars of yoga: Yama (Universal moral observances) and Niyama (Self-discipline) 21.
Throughout the session, the therapist will encourage patients to accept themselves, their limitations, and their values.
The session will then progress to a series of chair-based Asanas (Physical postures) for the neck, back, and limbs, synchronized with the same breathing rhythm.
This aspect of yoga is widely recognized [14] , [19].
To mobilize the cervical spine, patients will be guided to gently tilt their head from side to side and perform slow clockwise and counterclockwise rotations.
Trunk mobility will be addressed by placing one hand on the opposite knee and the other on the chair's back while twisting.
For lower limb mobilization, patients will perform seated knee flexion and extension movements and practice maintaining balance in single-leg positions with hands in the air [14], [19], [28].
The last five minutes will include Pratyahara (Sense withdrawal) and Dharana (Concentration) and finished by Dhyana (Meditation) and Samadhi (Absorption) 27, 42 .
Samadhi represents a state of profound bliss or enlightenment that promotes self-acceptance and enhances self-esteem 27, 42, 43 .
During the meditation phase, patients will be instructed to close their eyes, take deep diaphragmatic breaths, relax their neck, shoulders, hands, and toes, release negative thoughts with each exhalation, and welcome positive thoughts while accepting their illness.
Finally, the therapist will prompt patients to acknowledge their accomplishments, express gratitude, and emphasize the importance of self-love [46].
RM and HH will supervise the intervention, the unintended events, and will decide who will stop and continue the intervention.
Statistical methods
Quantitative data distribution will be assessed using the Kolmogorov-Smirnov test.
For normally distributed data with equal variances, results will be presented as mean ± SD.
Non-normally distributed data will be presented as median(interquartile range).
Categorical data will be presented as frequencies and percentages (%).
Student’s T-test or Mann-Whitney U test will compare quantitative data between groups, while the Chi-square test will compare categorical data.
The interventional program will be deemed clinically effective if it produces a change in verbal fluency exceeding the recommended MCID of two words in the NPI-Q score compared to the initial assessment 29, 30.
Expected Results
The outcomes expected from the results of this protocol are:
i) Enhance number of words due to concentration pillar of “" Yoga-like”.
ii) Improving execution speed, problem solving, memory and visuo-spatial control due postural pillars and spiritual pillars of “" Yoga-like”.
iii) Improving mood due meditation parts.
Declaration
The authors wish to disclose that an artificial intelligence tool (ie; ChatGPT 3.5) was utilized to enhance the clarity and coherence of the manuscript' writing.
The tool was utilized for language refinement purposes only, ensuring the text was clear and coherent without altering the scientific content or generating any new text [50].
References
- Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- García-Morales V, González-Acedo A, Melguizo-Rodríguez L, Pardo-Moreno T, Costela-Ruiz VJ, Montiel-Troya M, et al. Current understanding of the physiopathology, diagnosis and therapeutic approach to Alzheimer’s. Biomedicines. 2021;9(12):1910. doi: 10.3390/biomedicines9121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias NS, Barbosa IG, Kuang W, Teixeira AL. Depressive disorders in the elderly and dementia: An update. Dement Neuropsychol. 2020;14(1):1–6. doi: 10.1590/1980-57642020dn14-010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Booth A, Rockwood K, Peters J, D'Este C, Anstey KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022846. doi: 10.1136/bmjopen-2018-022846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letra L, Santana I, Seiça R. Obesity as a risk factor for Alzheimer's disease: the role of adipocytokines. Metab Brain Dis. 2014;29(3):563–568. doi: 10.1007/s11011-014-9501-z. [DOI] [PubMed] [Google Scholar]
- Vaz M, Silvestre S. Alzheimer's disease: Recent treatment strategies. Eur J Pharmacol. 2020;887:173554. doi: 10.1016/j.ejphar.2020.173554. [DOI] [PubMed] [Google Scholar]
- Passeri E, Elkhoury K, Morsink M, Broersen K, Linder M, Tamayol A, et al. Alzheimer's disease: Treatment strategies and their limitations. Int J Mol Sci. 2022;23(22):13954. doi: 10.3390/ijms232213954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: An update. J Cent NervSyst Dis. 2020;12:1179573520907397. doi: 10.1177/1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KenneMalaha A, Thébaut C, Achille D, Preux PM, Guerchet M. Costs of dementia in low- and middle-income countries: A systematic review. J Alzheimers Dis. 2023;91(1):115–128. doi: 10.3233/JAD-220239. [DOI] [PubMed] [Google Scholar]
- Ben Ayed I, Castor-Guyonvarch N, Amimour S, Naija S, Aouichaoui C, Ben Omor S. Acute exercise and cognitive function in Alzheimer's disease. J Alzheimers Dis. 2021;82(2):749–760. doi: 10.3233/JAD-201317. [DOI] [PubMed] [Google Scholar]
- Fonte C, Smania N, Pedrinolla A, Munari D, Gandolfi M, Picelli A. Comparison between physical and cognitive treatment in patients with MCI and Alzheimer's disease. Aging (Albany NY) 2019;11(10):3138–3155. doi: 10.18632/aging.101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne RA, Aarsland D, O'Brien JT, Ballard C, Banerjee S, Fox NC. Mild cognitive impairment: the Manchester consensus. Age Ageing. 2021;50(1):72–80. doi: 10.1093/ageing/afaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murroni V, Cavalli R, Basso A, Borella E, Meneghetti C, Melendugno A. Effectiveness of therapeutic gardens for people with dementia: A systematic review. Int J Environ Res Public Health. 2021;18(18):9595. doi: 10.3390/ijerph18189595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobe S, Chobe M, Metri K, Patra SK, Nagaratna R. Impact of Yoga on cognition and mental health among elderly: A systematic review. Complement Ther Med. 2020;52:102421. doi: 10.1016/j.ctim.2020.102421. [DOI] [PubMed] [Google Scholar]
- Merom D, Cumming R, Mathieu E, Anstey KJ, Rissel C, Simpson JM, et al Can social dancing prevent falls in older adults? A protocol of the Dance, Aging, Cognition, Economics (DAnCE) fall prevention randomised controlled trial. BMC Public Health. 2013;13:477. doi: 10.1186/1471-2458-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkou V, Aithal S, Richards M, Hiley E, Meekums B. Dance movement therapy for dementia. Cochrane Database Syst Rev. 2023;8(8) doi: 10.1002/14651858.CD011022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira TC, Soares FC, De Macedo LD, Diniz DL, Bento-Torres NV, Picanço-Diniz CW. Beneficial effects of multisensory and cognitive stimulation on age-related cognitive decline in long-term-care institutions. Clin Interv Aging. 2014;9:309–320. doi: 10.2147/CIA.S54383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Davis J, Ott BR, Uebelacker L, Kenney L, Gillette T, Britton K, Sanborn V. Feasibility of a yoga intervention for individuals with mild cognitive impairment: A randomized controlled trial. J Integr Complement Med. 2022;28(3):250–260. doi: 10.1089/jicm.2021.0204. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Siddarth P, Acevedo B, Van Dyk K, Paholpak P, Ercoli L, St Cyr N, et al A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557–567. doi: 10.1017/S1041610216002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzenda A, Siddarth P, Milillo MM, Aguilar-Faustino Y, Khalsa DS, Lavretsky H. Cognitive and immunological effects of yoga compared to memory training in older women at risk for Alzheimer's disease. Transl Psychiatry. 2024;14(1):96. doi: 10.1038/s41398-024-02807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt S, Barnable T. Definition of a Yoga breathing (Pranayama) protocol that improves lung function. Holist Nurs Pract. 2019;33(4):197–203. doi: 10.1097/HNP.0000000000000331. [DOI] [PubMed] [Google Scholar]
- Villemure C, Čeko M, Cotton VA, Bushnell MC. Neuroprotective effects of yoga practice: age-, experience-, and frequency dependent plasticity. Front Hum Neurosci. 2015;9:281. doi: 10.3389/fnhum.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe NP, Khan I, Hayes J, Erlenbach E, Damoiseaux JS. Yoga effects on brain health: A systematic review of the current literature. Brain Plast. 2019;5(1):105–122. doi: 10.3233/BPL-190084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S, Cerna J, Gothe NP. Yoga impacts cognitive health: Neurophysiological changes and stress-regulation mechanisms. Exerc Sport Sci Rev. 2023;51:73–81. doi: 10.1249/JES.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamacoska D, Tan T, Mathersul DC, Sabag A, de Manincor M, Chang D, Steiner-Lim GZ. A systematic review of the health effects of yoga for people with mild cognitive impairment and dementia. BMC Geriatr. 2023;23(1):37. doi: 10.1186/s12877-023-03732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann HC, Vennemann J, Gross J, Matko K, Sedlmeier P. "To be finally at peace with myself": A qualitative study reflecting experiences of the meditation-based lifestyle modification program in mild-to-moderate depression. J Altern Complement Med. 2021;27(9):786–795. doi: 10.1089/acm.2021.0038. [DOI] [PubMed] [Google Scholar]
- Csala B, Springinsfeld CM, Köteles F. The relationship between Yoga and spirituality: A systematic review of empirical research. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.695939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Iglesias D, García-Porro M, Clardy A, Ayán Pérez C. Feasibility and effects of a chair-based yoga program for adults with neurodisability. Disabil Rehabil. 2022;44(18):5220–5230. doi: 10.1080/09638288.2021.1933617. [DOI] [PubMed] [Google Scholar]
- Mao HF, Kuo CA, Huang WN, Cummings JL, Hwang TJ. Values of the minimal clinically important difference for the neuropsychiatric inventory questionnaire in individuals with dementia. J Am Geriatr Soc. 2015;63(7):1448–1452. doi: 10.1111/jgs.13473. [DOI] [PubMed] [Google Scholar]
- Feghali Y, Fares Y, Abou Abbas L. Assessment of neuropsychiatric symptoms in dementia: validity and reliability of the Lebanese version of the neuropsychiatric inventory questionnaire. Appl Neuropsychol Adult. 2021;28(5):588–595. doi: 10.1080/23279095.2019.1670182. [DOI] [PubMed] [Google Scholar]
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža Jerić K. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhier Z, Bendahhou K, Ben Abdelaziz A, Bennani MO. Methodological sheet n°1: How to calculate the size of a sample for an observational study? Tunis Med. 2020;98(1):1–7. [PubMed] [Google Scholar]
- Brodaty H, Connors MH, Xu J, Woodward M, Ames D, PRIME study group The course of neuropsychiatric symptoms in dementia: a 3-year longitudinal study. J Am Med Dir Assoc. 2015;16(5):380–387. doi: 10.1016/j.jamda.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Albanna M, Yehya A, Khairi A, Dafeeah E, Elhadi A, Rezgui L. Validation and cultural adaptation of the Arabic versions of the mini mental status examination - 2 and mini-cog test. Neuropsychiatr Dis Treat. 2017;13:793–801. doi: 10.2147/NDT.S126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar R, Tripathi R, Bharath S, Kumar K. Classic tower of Hanoi, planning skills, and the Indian elderly. East Asian Arch Psychiatry. 2015;25(3):108–114. [PubMed] [Google Scholar]
- Scarpina F, Tagini S. The stroop color and word test. Front Psychol. 2017;8:557. doi: 10.3389/fpsyg.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghatani A, Obonsawin M, Al-Moutaery K. The Arabic version of the Stroop Test and its equivalency to the English version. Pan Arab J Neurosurg. 2010;14:112–115. [Google Scholar]
- Chaaya M, Sibai AM, Roueiheb ZE, Chemaitelly H, Chahine LM, Al-Amin H, Mahfoud Z. Validation of the Arabic version of the short geriatric depression scale (GDS-15). Int Psychogeriatr. 2008;20(3):571–581. doi: 10.1017/S1041610208006741. [DOI] [PubMed] [Google Scholar]
- Schmid AA, Sternke EA, Do AL, Conner NS, Starnino VR, Davis LW. The eight limbs of yoga can be maintained in a veteran friendly yoga program. Int J Yoga. 2021;14(2):127–132. doi: 10.4103/ijoy.IJOY_106_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibben E. Mind-stuff and withdrawal of the senses: Toward an interpretation of pratyahara in contemporary postural yoga. Health (London) 2024 doi: 10.1177/13634593231222450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telles S, Desiraju T. Autonomic changes in Brahmakumaris Raja yoga meditation. Int J Psychophysiol Off J Int Organ Psychophysiol. 1993;15:147–152. doi: 10.1016/0167-8760(93)90072-w. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Martarelli D, Cocchioni M, Scuri S, Pompei P. Diaphragmatic breathing reduces exercise-induced oxidative stress. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1093/ecam/nep169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso JC, McHale A, Ende V, Oberlin DJ, Suzuki WA. Brief, daily meditation enhances attention, memory, mood, and emotional regulation in non-experienced meditators. Behav Brain Res. 2019;356:208–220. doi: 10.1016/j.bbr.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Alam KK, Gangwani N, Mohan M. Perception and practice of the eight limbs of yoga in yoga teachers: A cross-sectional descriptive study. J Fam Med Prim Care. 2024;13:1500–1506. doi: 10.4103/jfmpc.jfmpc_1711_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl SJ, Brenes GA, Krucoff C, Hargis G, Anderson A, Miller ME, Danhauer SC. Ensuring yoga intervention fidelity in a randomized preference trial for the treatment of worry in older adults. J Altern Complement Med. 2021;27(6):489–495. doi: 10.1089/acm.2020.0476. [DOI] [PubMed] [Google Scholar]
- Kannan P, Hsu WH, Suen WT, Chan LM, Assor A, Ho CM. Yoga and Pilates compared to pelvic floor muscle training for urinary incontinence in elderly women: A randomised controlled pilot trial. Complement Ther Clin Pract. 2022;46 doi: 10.1016/j.ctcp.2021.101502. [DOI] [PubMed] [Google Scholar]
- Dergaa I, Ben Saad H. Artificial intelligence and promoting open access in academic publishing. Tunis Med. 2023;101(6):533–536. [PMC free article] [PubMed] [Google Scholar]


