Abstract
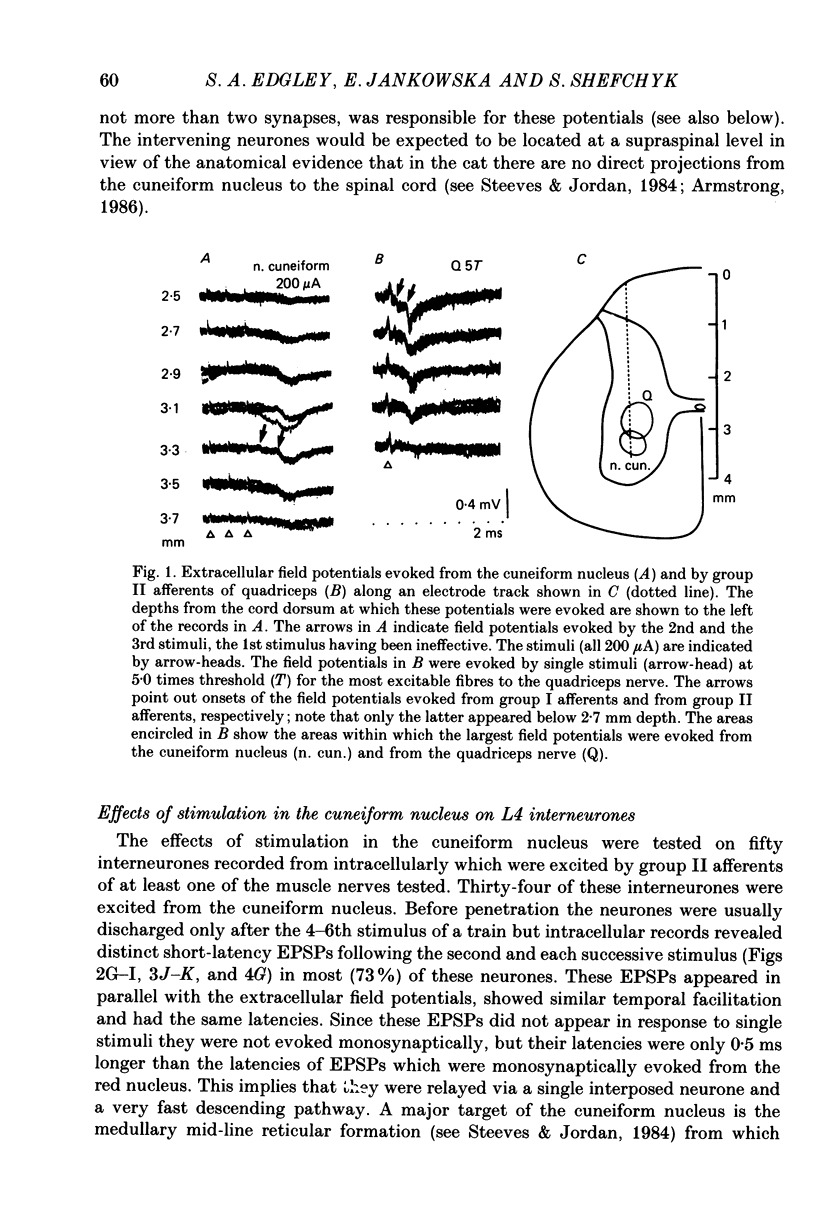
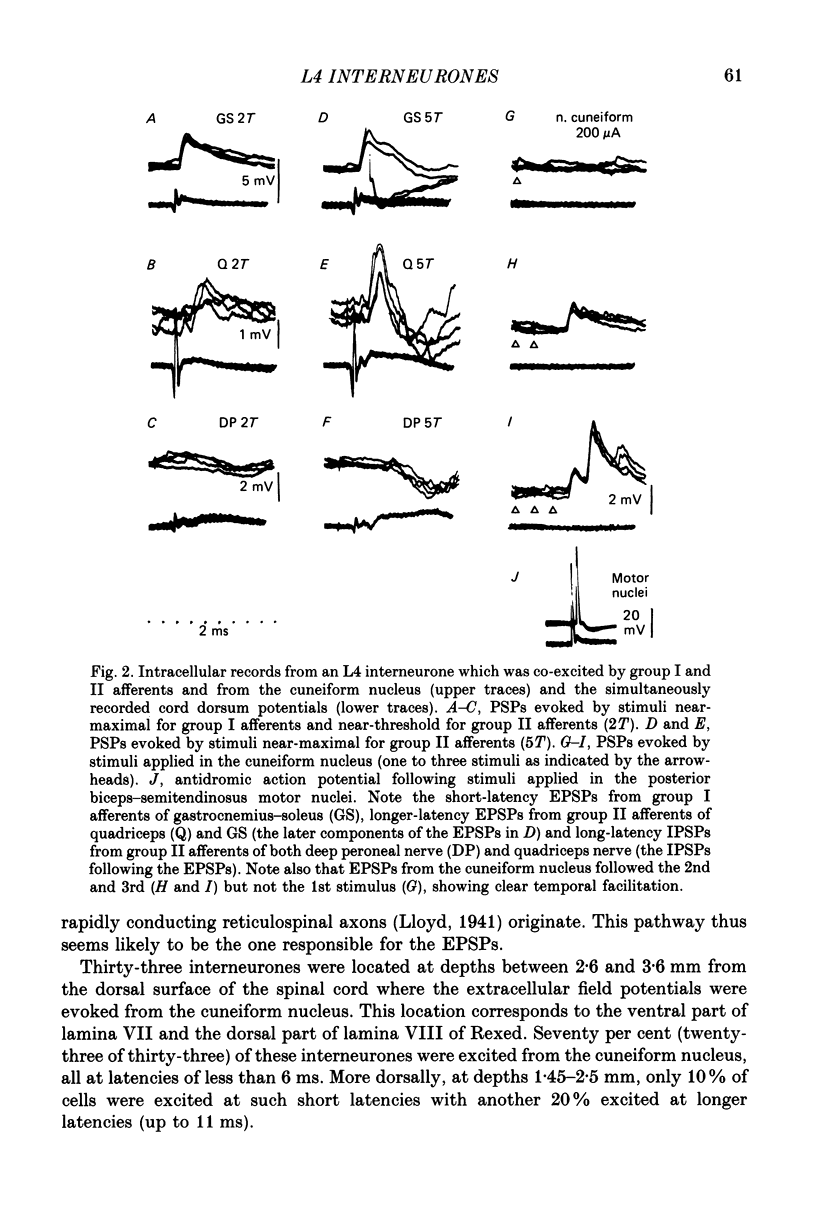
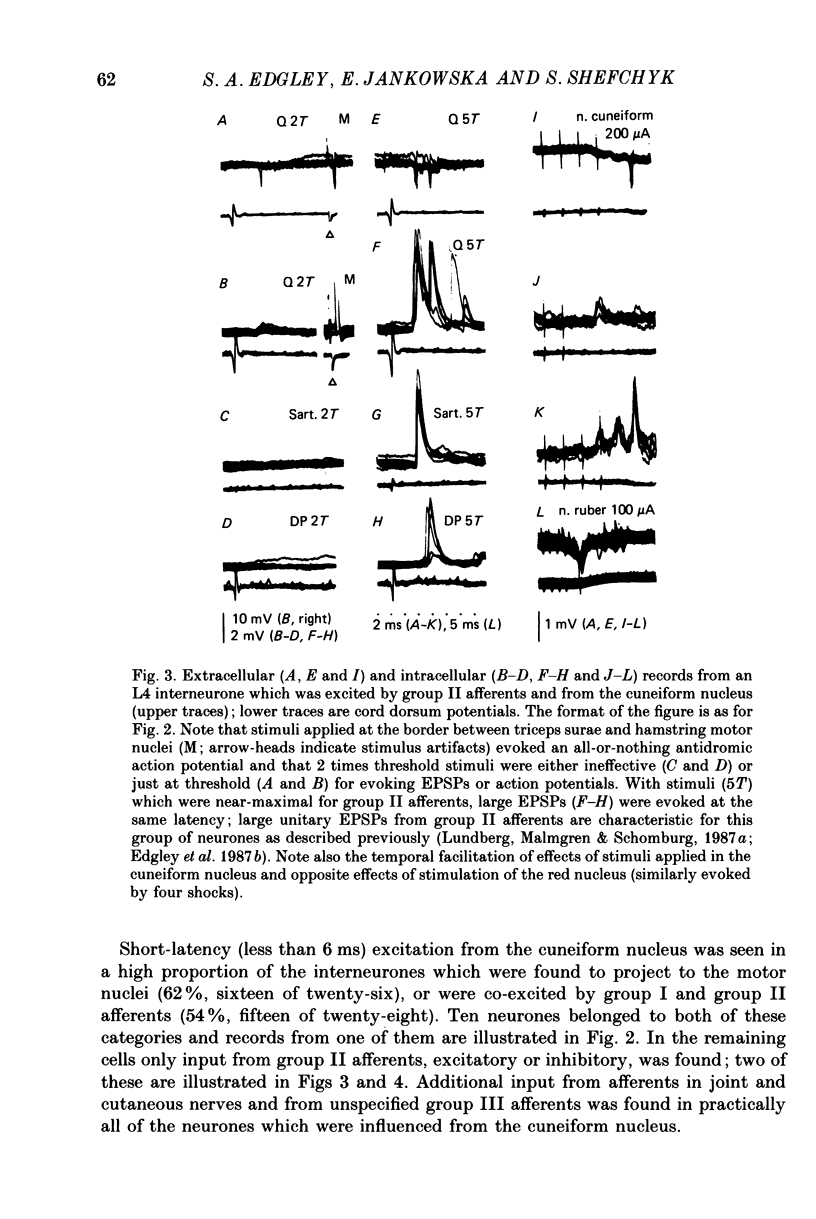
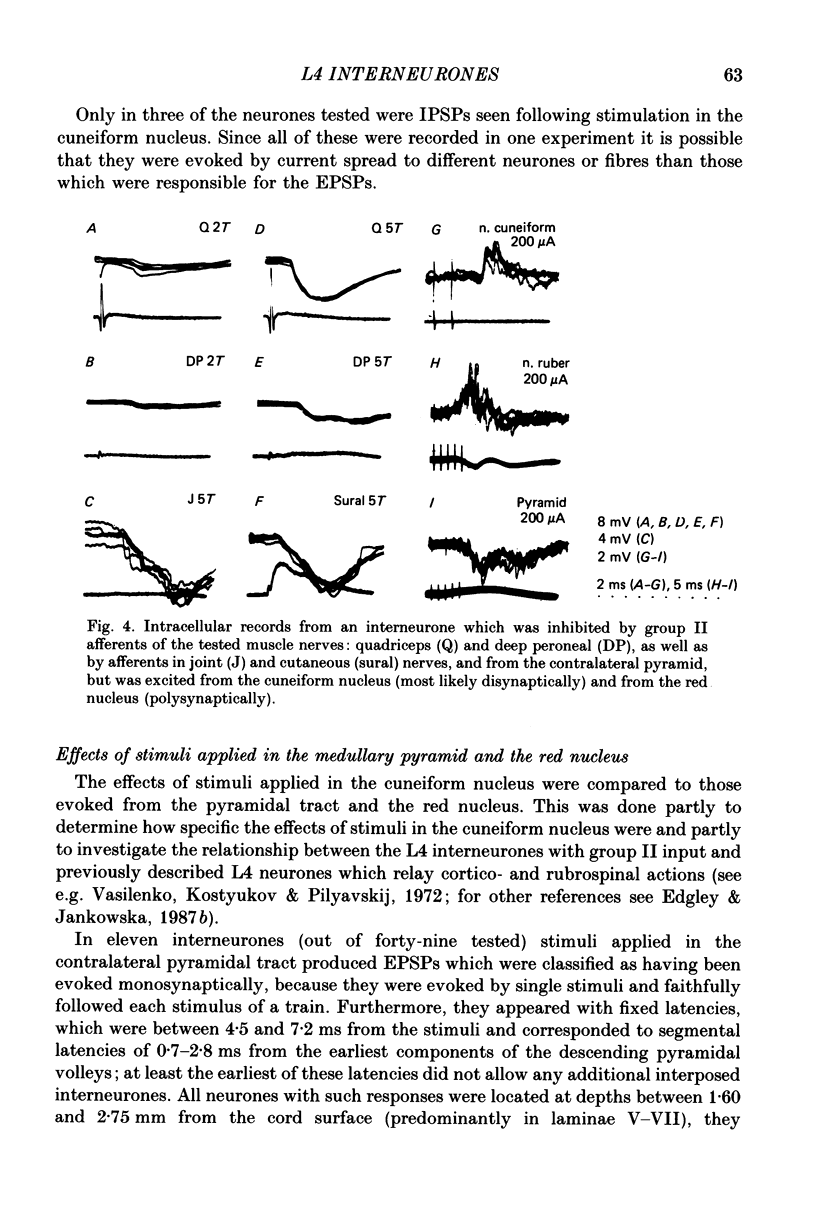
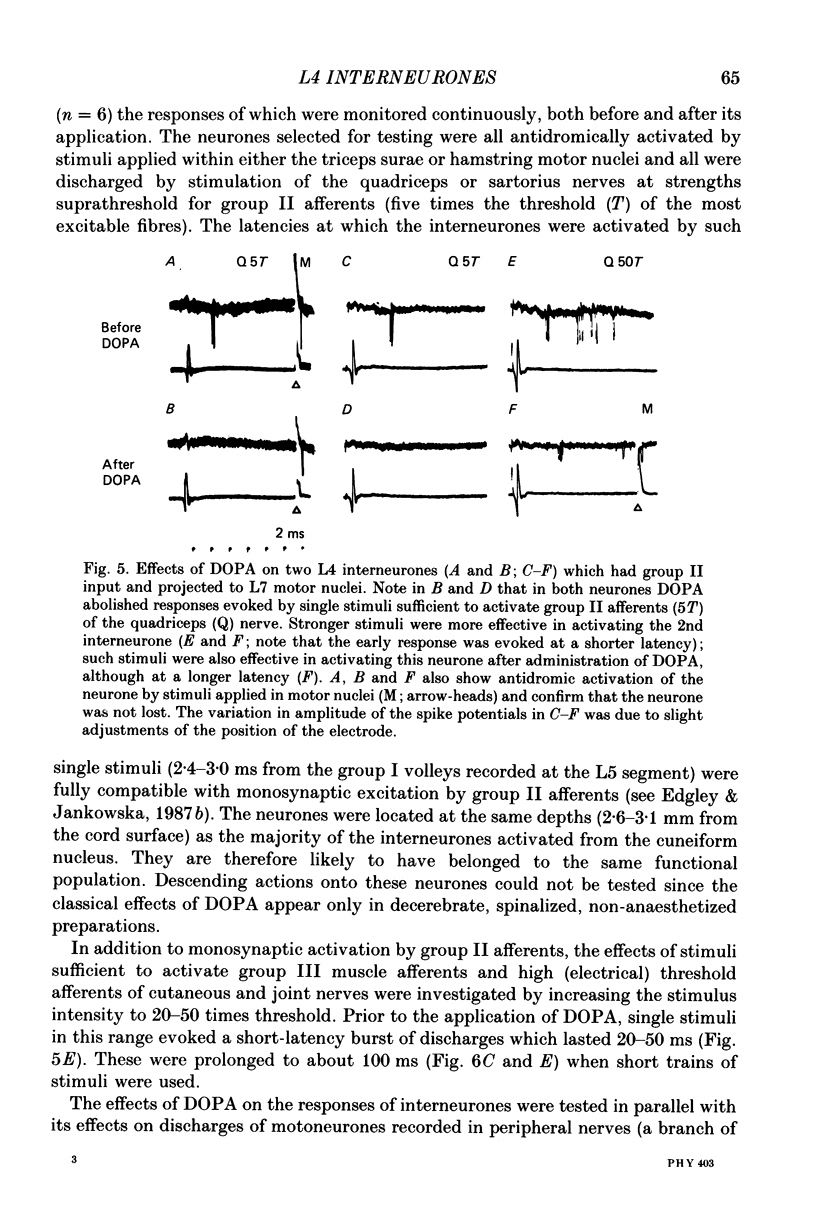
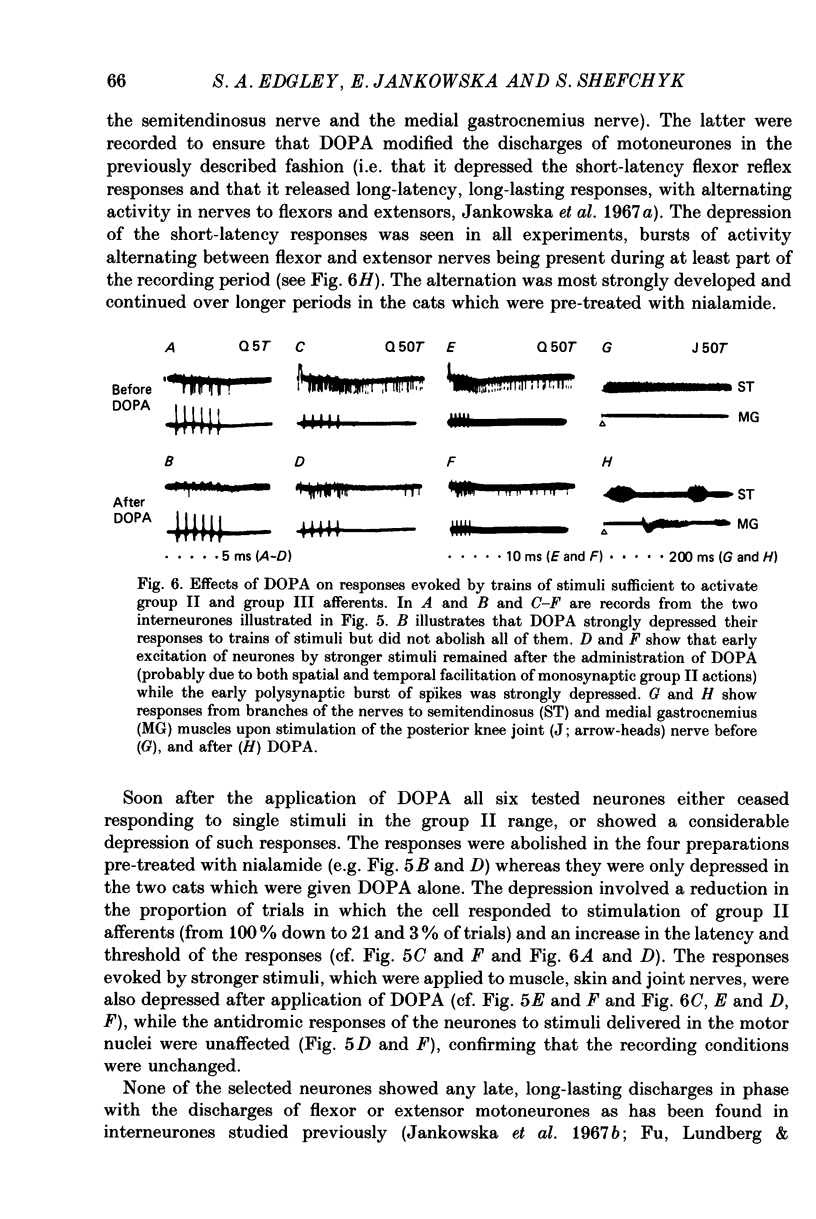
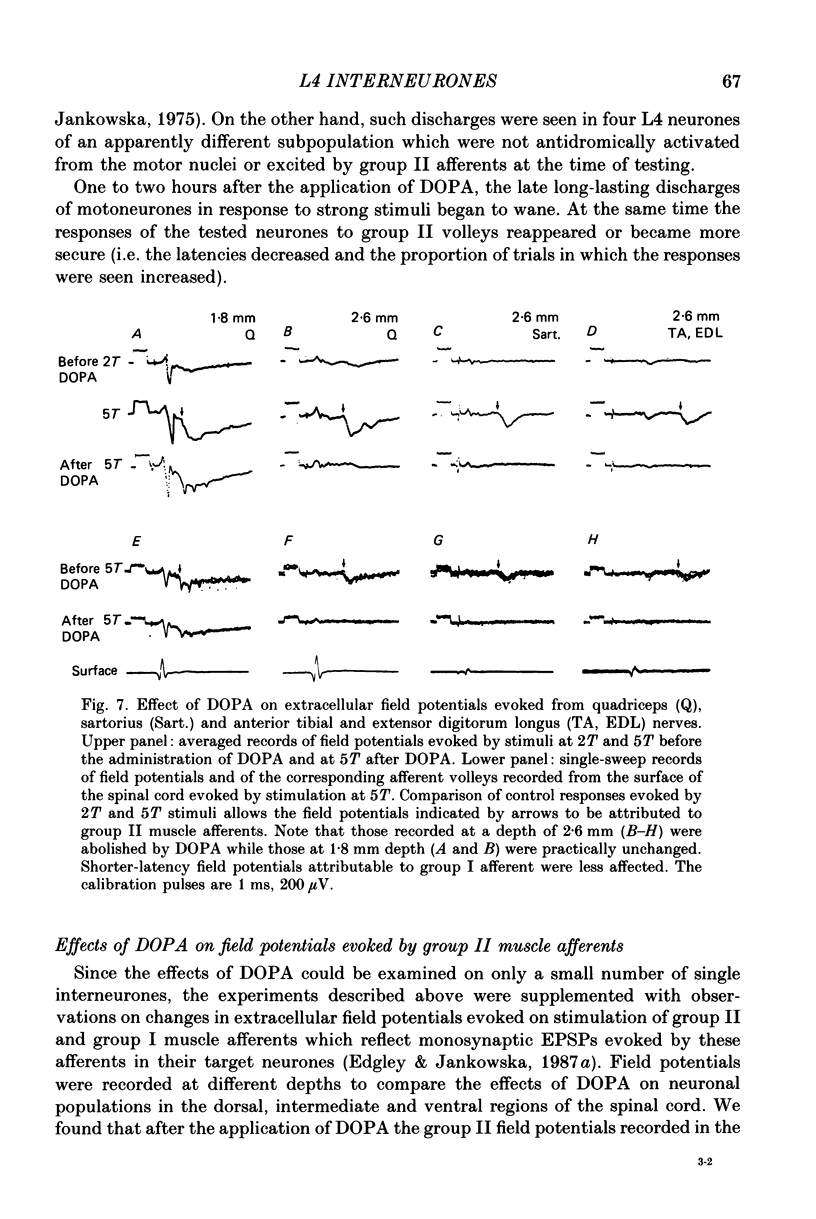
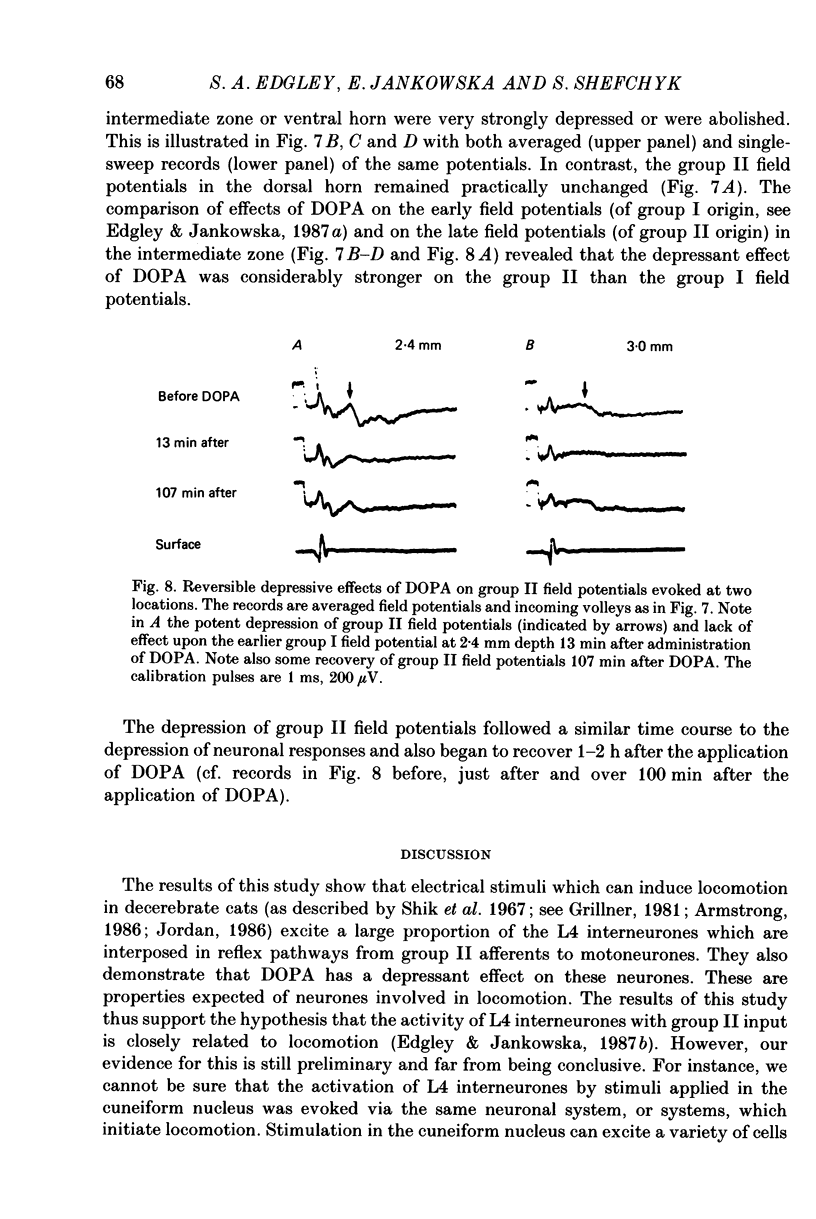
1. A group of interneurons in the mid-lumbar segments of the cat spinal cord which mediate disynaptic excitation or inhibition of motoneurones from group II muscle afferents have recently been described. To test the possibility that the activity of these interneurones is related to the activity in the neuronal networks which subserve locomotion we have investigated whether they are influenced by two procedures which can induce locomotion. These procedures were electrical stimulation within the cuneiform nucleus (the 'mesencephalic locomotor region') in anaesthetized preparations and systemic administration of 3,4-dihydroxyphenylalanine (DOPA) in decerebrate, spinalized, unanaesthetized preparations. The interneurones we have tested were located in the fourth lumbar (L4) segment and were excited by group II muscle afferents; more than half of them were antidromically activated from the hindlimb motor nuclei. 2. Stimuli applied in the cuneiform nucleus evoked excitatory postsynaptic potentials (EPSPs) in a high proportion of these interneurones. The stimuli also evoked distinct extracellular field potentials in the ventral horn of the L4 segment. The properties and latencies of both the intra- and extracellularly recorded potentials show that they were evoked disynaptically, via supraspinally located relay neurones and a fast-conducting descending tract. 3. Stimulation of the cortico- and rubrospinal tracts excited or inhibited some of the L4 neurones, often at latencies suggesting mono- or disynaptic coupling. The neurones which appeared to be monosynaptically excited from the cortico- and rubrospinal tracts tended to be located dorsal to the neurones which were activated from the cuneiform nucleus. 4. Systemic administration of DOPA depressed the responses evoked by stimulation of group II afferents of L4 interneurones which projected to motor nuclei. DOPA also depressed extracellular field potentials evoked by group II afferents in the intermediate zone and in the ventral horn (at the location of the interneurones) but hardly affected those in the dorsal horn. 5. By showing that both stimulation in the cuneiform nucleus and the administration of DOPA influence activity of L4 interneurones which are excited by group II afferents and which project to motor nuclei, the results of this study support the hypothesis that these neurones are in some way involved in locomotion. However, the opposing effects of DOPA administration and of stimulation in the cuneiform nucleus make the interpretation of their role in locomotion rather difficult before it is known to what extent they are active throughout the step cycle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26(4):273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Berkinblit M. B., Deliagina T. G., Feldman A. G., Gelfand I. M., Orlovsky G. N. Generation of scratching. I. Activity of spinal interneurons during scratching. J Neurophysiol. 1978 Jul;41(4):1040–1057. doi: 10.1152/jn.1978.41.4.1040. [DOI] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina T. G., Orlovsky G. N., Pavlova G. A. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res. 1983;53(1):81–90. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7(4):344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15(1):54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Fu T. C., Lundberg A. Reciprocal La inhibition during the late reflexes evoked from the flexor reflex afferents after DOPA. Brain Res. 1975 Feb 21;85(1):99–102. doi: 10.1016/0006-8993(75)91012-4. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967 Jul-Aug;70(3):369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967 Jul-Aug;70(3):389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Exp Brain Res. 1987;65(2):282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987;65(2):294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Mori S., Shik M. L., Yagodnitsyn A. S. Role of pontine tegmentum for locomotor control in mesencephalic cat. J Neurophysiol. 1977 Mar;40(2):284–295. doi: 10.1152/jn.1977.40.2.284. [DOI] [PubMed] [Google Scholar]
- Severin F. V., Shik M. L., Orlovskii G. N. Rabota myshts i odinochnykh motoneironov pri upravliamoi lokomotsii. Biofizika. 1967 Jul-Aug;12(4):660–668. [PubMed] [Google Scholar]
- Shefchyk S. J., Jell R. M., Jordan L. M. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56(2):257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- Shefchyk S. J., Jordan L. M. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985 Jun;53(6):1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Steeves J. D., Jordan L. M. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 1984 Jul 30;307(1-2):263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]


