Abstract
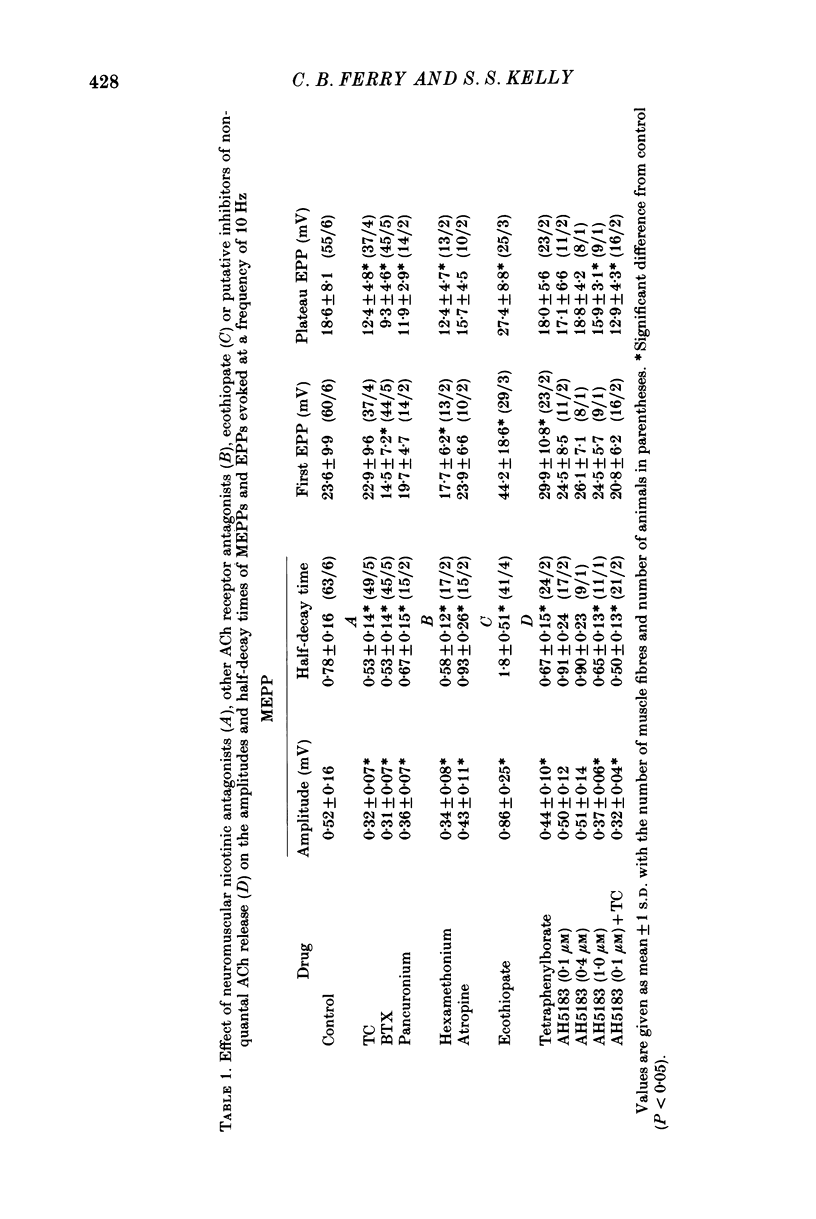
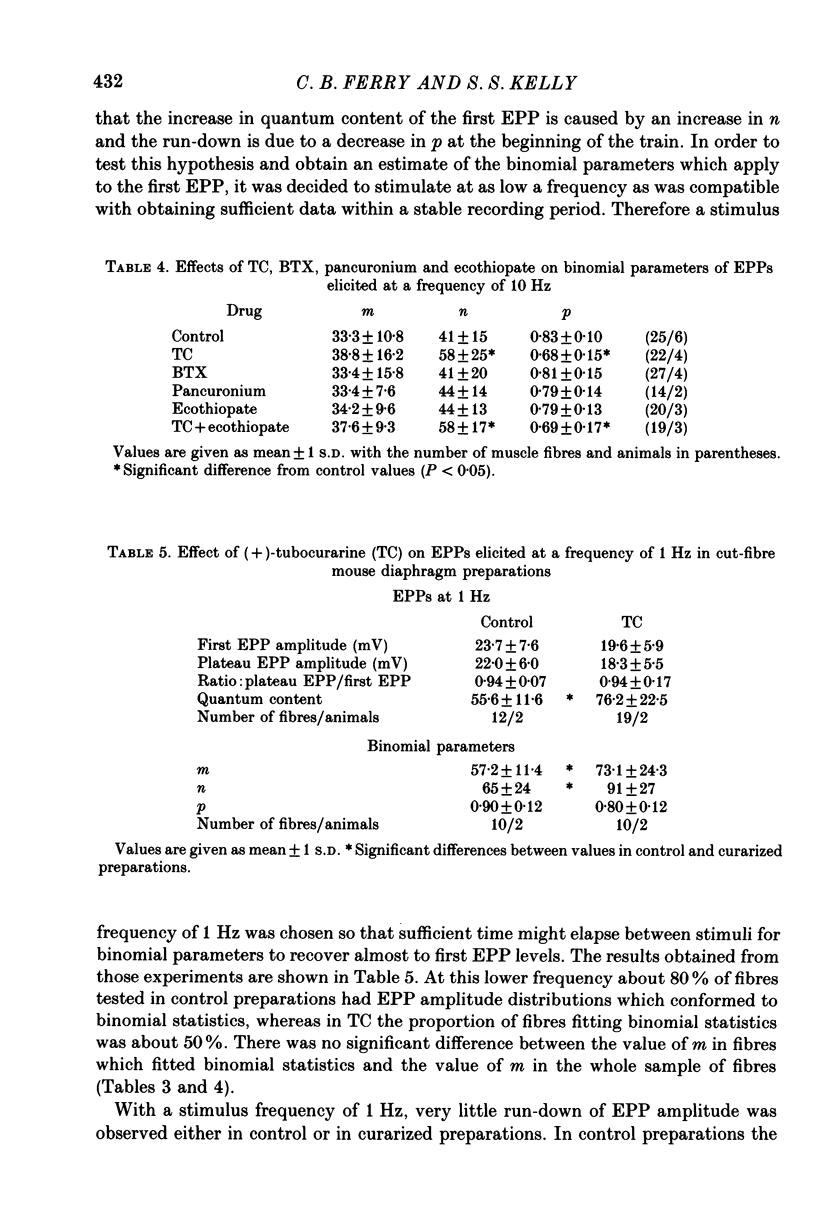
1. The effects of (+)-tubocurarine (TC) on tetanic run-down and quantum content of end-plate potentials (EPPs) were investigated in cut-fibre preparations of mouse diaphragm. 2. (+)-Tubocurarine, 0.15 microM, halved the amplitude of spontaneous miniature EPPs (MEPPs) and steepened the tetanic run-down of EPPs evoked at 10 Hz by increasing the quantum content of the first EPP of the train while having no effect on quantum content of plateau EPPs. With stimulation at 1 Hz, there was little run-down and the quantum content of all EPPs was increased by TC. 3. The use of binomial statistics to analyse release indicated that after TC the increase in the quantum content of the first EPP in the train at 10 Hz was due to an increase in n and that during the run-down there was a decrease in p so that plateau EPP quantum content at 10 Hz was not different from control. 4. To elucidate a possible role of cholinoreceptors in the presynaptic effects of TC, studies were made on the effects of pancuronium or of alpha-bungarotoxin (BTX), with concentrations and exposure times where they had postsynaptic effects equal to 0.15 microM-TC. The run-down of EPPs was unaffected by BTX, while pancuronium steepened it to a lesser extent than TC. 5. The anticholinesterase, ecothiopate, decreased the quantum content of plateau EPPs only at high frequencies of stimulation (50 Hz) and did not affect the presynaptic effects of TC at 10 Hz. 6. At concentrations which reduced MEPP amplitude, atropine (10 microM) or hexamethonium (50 microM) had no effect on EPP run-down. 7. These results indicate that TC could have presynaptic effects via a presynaptic acetylcholine receptor, but that such a receptor may not have the same binding specificities as the postsynaptic receptor.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Bierkamper G. G., Stanley E. F. Botulinum toxin prevents stimulus-induced backfiring produced by neostigmine in the mouse phrenic nerve-diaphragm. J Physiol. 1986 Mar;372:395–404. doi: 10.1113/jphysiol.1986.sp016015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- Banker B. Q., Kelly S. S., Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983 Jun;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber L. C. The mechanism of the facilitatory action of edrophonium in cat skeletal muscle. Br J Pharmacol. 1972 Nov;46(3):498–507. doi: 10.1111/j.1476-5381.1972.tb08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber L. C. The prejunctional actions of some non-depolarizing blocking drugs. Br J Pharmacol. 1973 Jan;47(1):109–116. doi: 10.1111/j.1476-5381.1973.tb08163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Hong S. J., Ko J. L. Mechanisms of the inhibition by neostigmine of tetanic contraction in the mouse diaphragm. Br J Pharmacol. 1986 Apr;87(4):757–762. doi: 10.1111/j.1476-5381.1986.tb14594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Dolezal V., Tucek S., Zemková H., Vyskocil F. Is an acetylcholine transport system responsible for nonquantal release of acetylcholine at the rodent myoneural junction? Proc Natl Acad Sci U S A. 1985 May;82(10):3514–3518. doi: 10.1073/pnas.82.10.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Curare and pancuronium compared: effects on previously undepressed mammalian myoneural junctions. Science. 1972 Nov 17;178(4062):753–755. doi: 10.1126/science.178.4062.753. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Schmidt R. F., Yokota T. The effect of acetylcholine upon mammalian motor nerve terminals. J Physiol. 1965 Dec;181(4):810–829. doi: 10.1113/jphysiol.1965.sp007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Statistics of neuromuscular transmitter release in young and old mouse muscle. J Physiol. 1987 Apr;385:507–516. doi: 10.1113/jphysiol.1987.sp016504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Mazurkiewicz J. E., Rosenthal J. Cytochemical localization of acetylcholine receptors at the neuromuscular junction by means of horseradish peroxidase-labeled alpha-bungarotoxin. Brain Res. 1977 Sep 2;132(3):423–442. doi: 10.1016/0006-8993(77)90192-5. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S., Terrar D. A. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981 Mar;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. D. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. J Physiol. 1975 Aug;250(1):121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D. The actions of cholinergic drugs on motor nerve terminals. Pharmacol Rev. 1977 Sep;29(3):221–247. [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. Influence of presynaptic receptors on neuromuscular transmission in rat. Am J Physiol. 1982 May;242(5):C366–C372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]


