Abstract
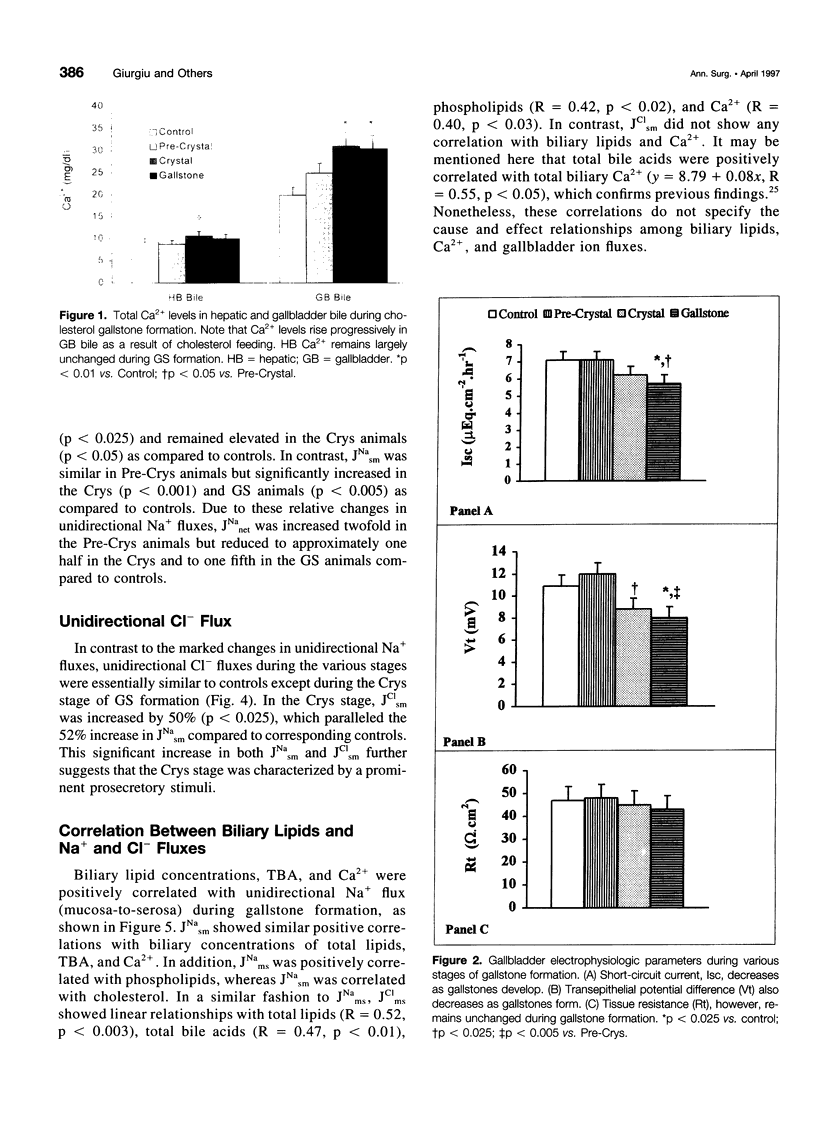
OBJECTIVE: This study sought to correlate gallbladder (GB) Na+ and Cl-) fluxes with biliary lipid composition during the various stages of gallstone (GS) formation. SUMMARY BACKGROUND DATA: GS formation is associated with altered GB ion transport and increased biliary lipid and Ca2+ concentrations. Nonetheless, the longitudinal relationship between ion transport and biliary lipid changes during GS formation has not been defined. METHODS: Prairie dogs were fed standard (n = 18) or 1.2% cholesterol-enriched (n = 30) diets for 4 to 21 days. Hepatic and GB bile were analyzed for lipids and Ca2+. Animals were designated either Pre-Crystal, Crystal, or GS based on absence or presence of crystals or GS, respectively. GBs were mounted in Ussing chambers, electrophysiologic parameters were recorded, and unidirectional Na+ and Cl- fluxes measured. RESULTS: Short-circuit current and potential difference were similar during Pre-Crystal and Crystal stages but significantly reduced during GS stage compared to controls and Pre-Crystals. Transepithelial resistance was similar in all groups. Net Na+ absorption was increased during Pre-Crystal but decreased during GS stage due to increased mucosa-to-serosa and serosa-to-mucosa flux, respectively. Increased serosa-to-mucosa flux of both Na+ and Cl- characterized the Crystal stage. Biliary lipids and Ca2+ increased progressively during various stages of GS formation and correlated positively with unidirectional fluxes of Na+ and Cl-. CONCLUSION: GB epithelial ion transport changes sequentially during GS formation, with the early Pre-Crystal stage characterized by increased Na+ absorption, and the later Crystal stage accompanied by prosecretory stimuli on Na+ and Cl- fluxes, which may be due to elevated GB bile Ca2+ and total bile acids.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abedin M. Z., Strichartz S. D., Festekdjian S., Roslyn J. J. Increased biliary calcium in cholesterol and pigment gallstone disease: the role of altered bile acid composition. Lipids. 1989 Jul;24(7):572–578. doi: 10.1007/BF02535071. [DOI] [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon H. V. Effect of taurine conjugated bile salts with and without lecithin on water and electrolyte transport in the canine gallbladder in vivo. Gastroenterology. 1979 Apr;76(4):778–783. [PubMed] [Google Scholar]
- Brenneman D. E., Connor W. E., Forker E. L., DenBesten L. The formation of abnormal bile and cholesterol gallstones from dietary cholesterol in the prairie dog. J Clin Invest. 1972 Jun;51(6):1495–1503. doi: 10.1172/JCI106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Cates J. A., Saunders K. D., Abedin M. Z., Roslyn J. J. The effects of extracellular calcium on prairie dog gallbladder ion transport. J Lab Clin Med. 1992 Dec;120(6):964–969. [PubMed] [Google Scholar]
- Connerty H. V., Briggs A. R. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966 Mar;45(3):290–296. doi: 10.1093/ajcp/45.3.290. [DOI] [PubMed] [Google Scholar]
- Conter R. L., Roslyn J. J., Porter-Fink V., DenBesten L. Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg. 1986 Jan;151(1):184–191. doi: 10.1016/0002-9610(86)90030-9. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Devor D. C., Sekar M. C., Frizzell R. A., Duffey M. E. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84) J Clin Invest. 1993 Nov;92(5):2173–2181. doi: 10.1172/JCI116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurll N., DenBesten L. Animal models of human cholesterol gallstone disease: a review. Lab Anim Sci. 1978 Aug;28(4):428–432. [PubMed] [Google Scholar]
- Harvey P. R., Somjen G., Gilat T., Gallinger S., Strasberg S. M. Vesicular cholesterol in bile. Relationship to protein concentration and nucleation time. Biochim Biophys Acta. 1988 Jan 19;958(1):10–18. doi: 10.1016/0005-2760(88)90240-8. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T. Animal models of cholesterol gallstone disease. Hepatology. 1984 Sep-Oct;4(5 Suppl):191S–198S. doi: 10.1002/hep.1840040836. [DOI] [PubMed] [Google Scholar]
- IWATA T., YAMASAKI K. ENZYMATIC DETERMINATION AND THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS IN BLOOD. J Biochem. 1964 Nov;56:424–431. doi: 10.1093/oxfordjournals.jbchem.a128013. [DOI] [PubMed] [Google Scholar]
- Kibe A., Dudley M. A., Halpern Z., Lynn M. P., Breuer A. C., Holzbach R. T. Factors affecting cholesterol monohydrate crystal nucleation time in model systems of supersaturated bile. J Lipid Res. 1985 Sep;26(9):1102–1111. [PubMed] [Google Scholar]
- Klinkspoor J. H., van Wijland M. J., Koeleman C. A., van Dijk W., Tytgat G. N., Groen A. K. Heterogeneity of human biliary mucin: functional implications. Clin Sci (Lond) 1994 Jan;86(1):75–82. doi: 10.1042/cs0860075. [DOI] [PubMed] [Google Scholar]
- Kochhar N., Kaul D. Molecular link between membrane cholesterol and Na+/H+ exchange within human platelets. FEBS Lett. 1992 Mar 24;299(1):19–22. doi: 10.1016/0014-5793(92)80090-4. [DOI] [PubMed] [Google Scholar]
- Lee S. P. Enhanced fluid transport across gallbladder mucosa in experimental cholelithiasis. Am J Physiol. 1978 Jun;234(6):E575–E578. doi: 10.1152/ajpendo.1978.234.6.E575. [DOI] [PubMed] [Google Scholar]
- Lee S. P., LaMont J. T., Carey M. C. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981 Jun;67(6):1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssac P. P., Bukhave K., Frederiksen O. Inhibitory effect of prostaglandins on isosmotic fluid transport by rabbit gall-bladder in vitro, and its modification by blocade of endogenous PGE-Biosynthesis with indomethacin. Acta Physiol Scand. 1974 Dec;92(4):496–507. doi: 10.1111/j.1748-1716.1974.tb05771.x. [DOI] [PubMed] [Google Scholar]
- Lijnen P., Fenyvesi A., Bex M., Bouillon R., Amery A. Erythrocyte cation transport systems and membrane lipids in insulin-dependent diabetes. Am J Hypertens. 1993 Sep;6(9):763–770. doi: 10.1093/ajh/6.9.763. [DOI] [PubMed] [Google Scholar]
- Malet P. F., Locke C. L., Trotman B. W., Soloway R. D. The calcium ionophore A23187 stimulates glycoprotein secretion by the guinea pig gallbladder. Hepatology. 1986 Jul-Aug;6(4):569–573. doi: 10.1002/hep.1840060404. [DOI] [PubMed] [Google Scholar]
- Moser A. J., Abedin M. Z., Cates J. A., Giurgiu D. I., Karam J. A., Roslyn J. J. Converting gallbladder absorption to secretion: the role of intracellular calcium. Surgery. 1996 Apr;119(4):410–416. doi: 10.1016/s0039-6060(96)80141-3. [DOI] [PubMed] [Google Scholar]
- Moser A. J., Abedin M. Z., Roslyn J. J. Increased biliary protein precedes gallstone formation. Dig Dis Sci. 1994 Jun;39(6):1313–1320. doi: 10.1007/BF02093799. [DOI] [PubMed] [Google Scholar]
- Nahrwold D. L., Rose R. C., Ward S. P. Abnormalities in gallbladder morphology and function in patients with cholelithiasis. Ann Surg. 1976 Oct;184(4):415–421. doi: 10.1097/00000658-197610000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady S. M., Wolters P. J. Sodium and chloride transport across the isolated porcine gallbladder. Am J Physiol. 1989 Jul;257(1 Pt 1):C45–C51. doi: 10.1152/ajpcell.1989.257.1.C45. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Gelarden R. T., Nahrwold D. L. Electrical properties of isolated human gallbladder. Am J Physiol. 1973 Jun;224(6):1320–1326. doi: 10.1152/ajplegacy.1973.224.6.1320. [DOI] [PubMed] [Google Scholar]
- Roslyn J. J., Abedin M. Z., Saunders K. D., Cates J. A., Strichartz S. D., Alperin M., Fromm M., Palant C. E. Uncoupled basal sodium absorption and chloride secretion in prairie dog (Cynomys ludovicianus) gallbladder. Comp Biochem Physiol A Comp Physiol. 1991;100(2):335–341. doi: 10.1016/0300-9629(91)90478-u. [DOI] [PubMed] [Google Scholar]
- Roslyn J. J., Abedin M. Z., Strichartz S. D., Abdou M. S., Palant C. E. Regulation of gallbladder ion transport: role of biliary lipids. Surgery. 1989 Feb;105(2 Pt 1):207–212. [PubMed] [Google Scholar]
- Roslyn J. J., Conter R. L., DenBesten L. Altered gallbladder concentration of biliary lipids during early cholesterol gallstone formation. Dig Dis Sci. 1987 Jun;32(6):609–614. doi: 10.1007/BF01296161. [DOI] [PubMed] [Google Scholar]
- Roslyn J. J., Doty J., Pitt H. A., Conter R. L., Den Besten L. Enhanced gallbladder absorption during gallstone formation: the roles of cholesterol saturated bile and gallbladder stasis. Am J Med Sci. 1986 Aug;292(2):75–80. doi: 10.1097/00000441-198608000-00002. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin S., Ståhlberg D., Einarsson K. Cholesterol metabolism in liver and gallbladder mucosa of patients with cholesterolosis. Hepatology. 1995 May;21(5):1269–1275. [PubMed] [Google Scholar]
- Sequeira S. S., Parkes H. G., Ellul J. P., Murphy G. M. In vitro determination by 1H-NMR studies that bile with shorter nucleation times contain cholesterol-enriched vesicles. Biochim Biophys Acta. 1995 Jun 6;1256(3):360–366. doi: 10.1016/0005-2760(95)00046-f. [DOI] [PubMed] [Google Scholar]
- Strichartz S. D., Abedin M. Z., Abdou M. S., Roslyn J. J. Increased biliary calcium in cholesterol gallstone formation. Am J Surg. 1988 Jan;155(1):131–137. doi: 10.1016/s0002-9610(88)80270-8. [DOI] [PubMed] [Google Scholar]
- Van Erpecum K. J., Stolk M. F., van den Broek A. M., Renooij W., van de Heijning B. J., van Berge Henegouwen G. P. Bile concentration promotes nucleation of cholesterol monohydrate crystals by increasing the cholesterol concentration in the vesicles. Eur J Clin Invest. 1993 May;23(5):283–288. doi: 10.1111/j.1365-2362.1993.tb00775.x. [DOI] [PubMed] [Google Scholar]





