Abstract
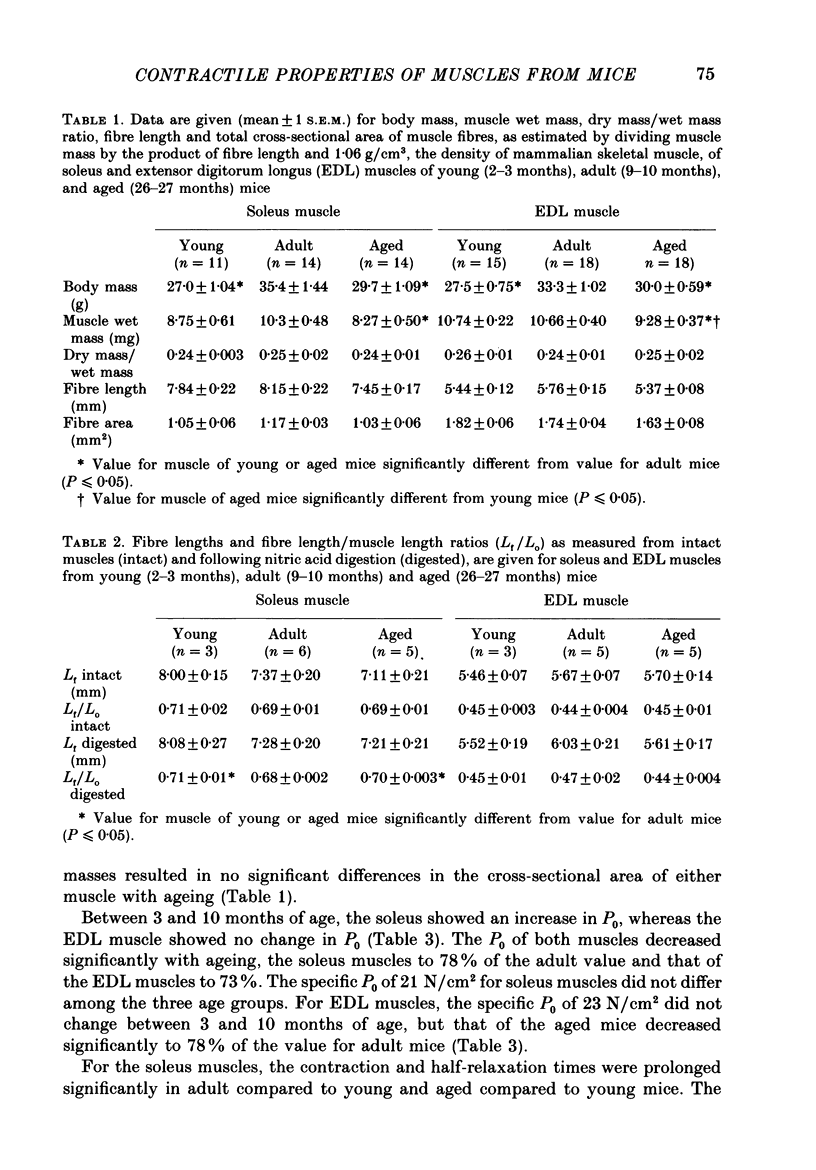
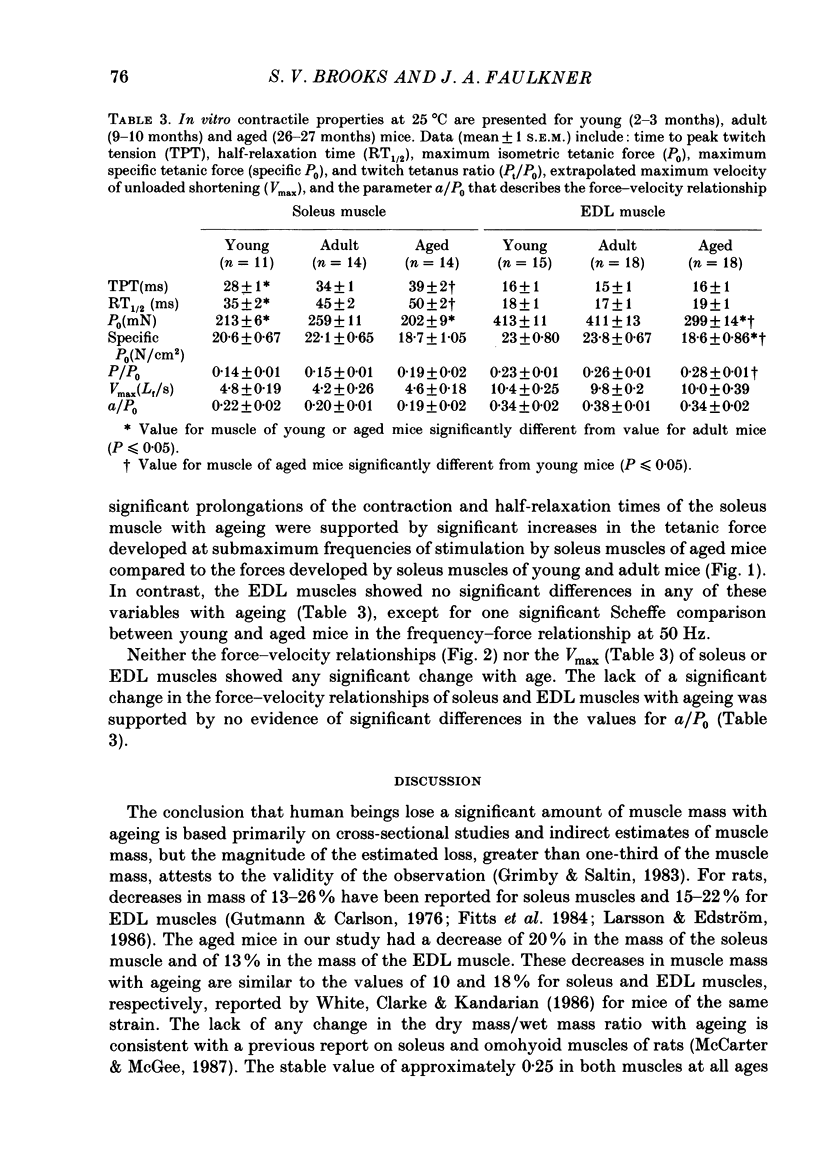
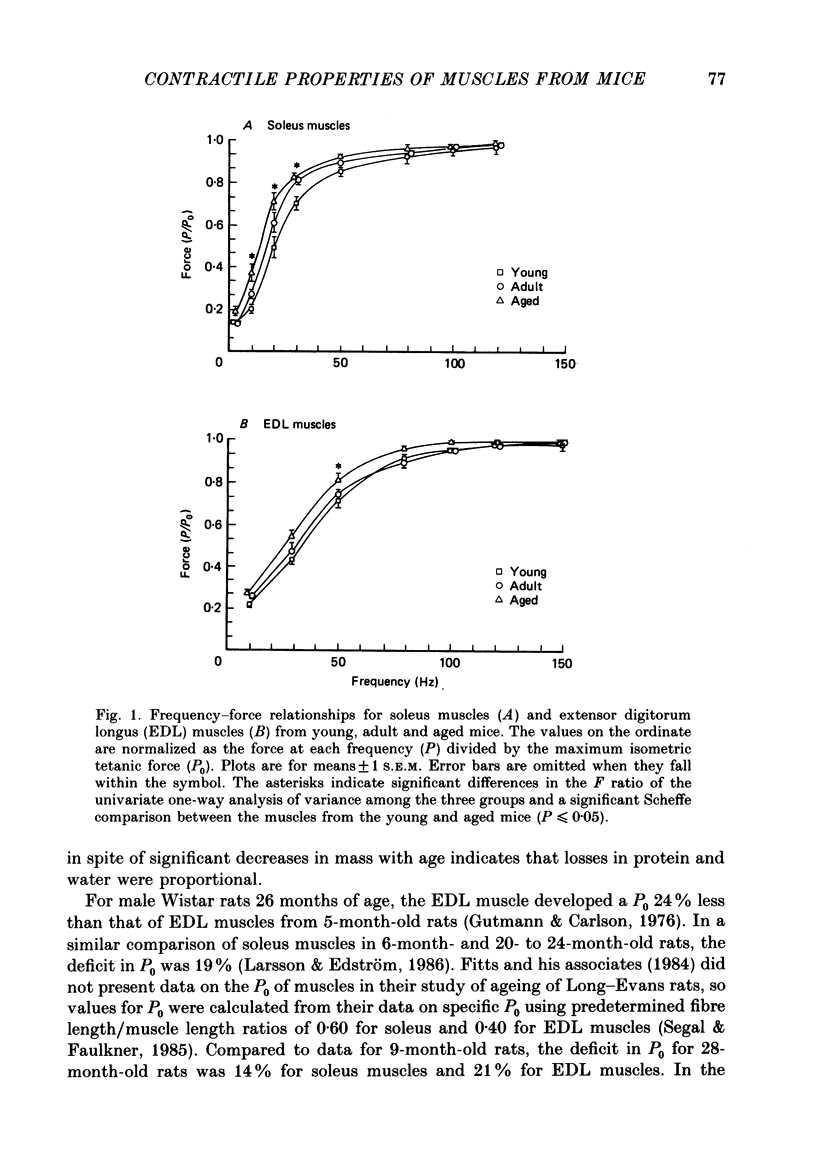
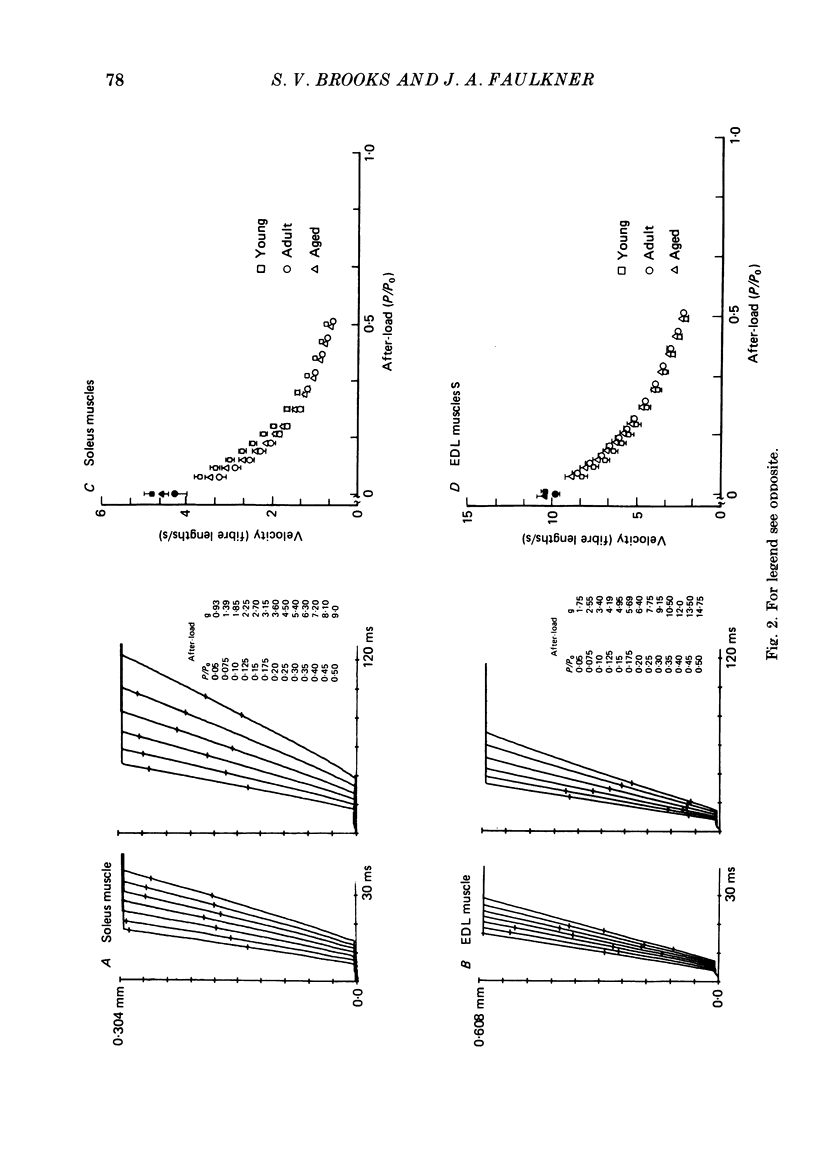
1. Comparisons were made in vitro at 25 degrees C among soleus and extensor digitorum longus (EDL) muscles from young (2-3 months), adult (9-10 months), and aged (26-27 months) male mice. We tested the hypotheses that, compared with soleus and EDL muscles of young and adult mice, those from aged mice develop decreased maximum tetanic force (P0, mN) and specific P0 (N/cm2), and that no significant differences occur for contraction time, half-relaxation time, or force-velocity relationship. 2. For the aged mice, the P0 of the soleus muscles and EDL muscles were 78 and 73% respectively of the values for adult mice. The specific P0 of EDL muscles of aged mice was 78% of the value of 23 N/cm2 obtained for young and adult mice. For soleus muscles, the specific P0 of 21 N/cm2 did not change with age. 3. Compared to values for young and adult mice, the contraction and half-relaxation times of soleus muscles from aged mice were increased, but the overall force-velocity relationships of soleus and EDL muscles did not change. The pooled values for the maximum velocity of unloaded shortening extrapolated from the force-velocity relationship of soleus and EDL muscles were 4.6 and 10.1 fibre lengths/s, respectively. 4. The decrease in the specific P0 of the EDL muscle with ageing must result from either a decrease in the number of cross-bridges in the driving stroke or a decrease in the force developed by each cross-bridge.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., LEWIS D. M. THE RATE OF TENSION DEVELOPMENT IN ISOMETRIC TETANIC CONTRACTIONS OF MAMMALIAN FAST AND SLOW SKELETAL MUSCLE. J Physiol. 1965 Feb;176:337–354. doi: 10.1113/jphysiol.1965.sp007554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. D., Krus D. L., Alizadeh B. Myosin degradation fragments in skeletal muscle. J Mol Biol. 1987 Jan 5;193(1):47–56. doi: 10.1016/0022-2836(87)90625-5. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R. H., Troup J. P., Witzmann F. A., Holloszy J. O. The effect of ageing and exercise on skeletal muscle function. Mech Ageing Dev. 1984 Oct 15;27(2):161–172. doi: 10.1016/0047-6374(84)90041-1. [DOI] [PubMed] [Google Scholar]
- Grimby G., Saltin B. The ageing muscle. Clin Physiol. 1983 Jun;3(3):209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Carlson B. M. Regeneration and transplantation of muscles in old rats and between young and old rats. Life Sci. 1976 Jan 1;18(1):109–114. doi: 10.1016/0024-3205(76)90280-0. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hoh J. Y., McGrath P. A., White R. I. Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick. Biochem J. 1976 Jul 1;157(1):87–95. doi: 10.1042/bj1570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Edström L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci. 1986 Nov;76(1):69–89. doi: 10.1016/0022-510x(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Larsson L., Grimby G., Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lännergren J. The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol. 1978 Oct;283:501–521. doi: 10.1113/jphysiol.1978.sp012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L. C., Faulkner J. A., Hyatt G. J. Estimation of number of fibers in guinea pig skeletal muscles. J Appl Physiol. 1974 Aug;37(2):259–264. doi: 10.1152/jappl.1974.37.2.259. [DOI] [PubMed] [Google Scholar]
- McCarter R., McGee J. Influence of nutrition and aging on the composition and function of rat skeletal muscle. J Gerontol. 1987 Jul;42(4):432–441. doi: 10.1093/geronj/42.4.432. [DOI] [PubMed] [Google Scholar]
- McCarter R., Radicke D., Yu B. P. A model preparation for studying fast mammalian skeletal muscles (39871). Proc Soc Exp Biol Med. 1977 Oct;156(1):40–45. doi: 10.3181/00379727-156-39871. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol. 1982 Aug;329:465–483. doi: 10.1113/jphysiol.1982.sp014314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity and myosin heavy chains of developing rabbit muscle fibers. J Biol Chem. 1985 Nov 25;260(27):14403–14405. [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985 Mar;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Segal S. S., White T. P., Faulkner J. A. Architecture, composition, and contractile properties of rat soleus muscle grafts. Am J Physiol. 1986 Mar;250(3 Pt 1):C474–C479. doi: 10.1152/ajpcell.1986.250.3.C474. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Gergely J., Sréter F. A. Velocity of shortening and myosin isozymes in two types of rabbit fast-twitch muscle fibers. Am J Physiol. 1986 Sep;251(3 Pt 1):C431–C434. doi: 10.1152/ajpcell.1986.251.3.C431. [DOI] [PubMed] [Google Scholar]
- Young A., Stokes M., Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984 Aug;14(4):282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Young A., Stokes M., Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985 Apr;5(2):145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]


