Abstract
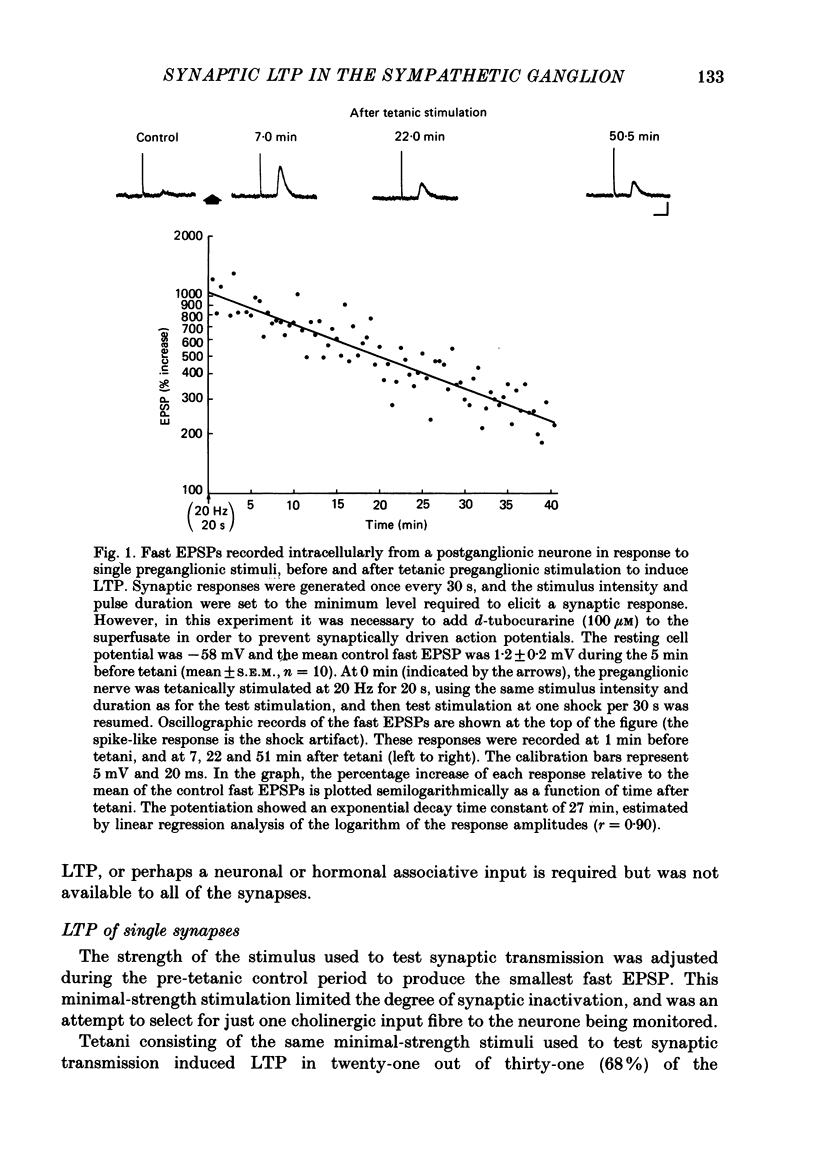
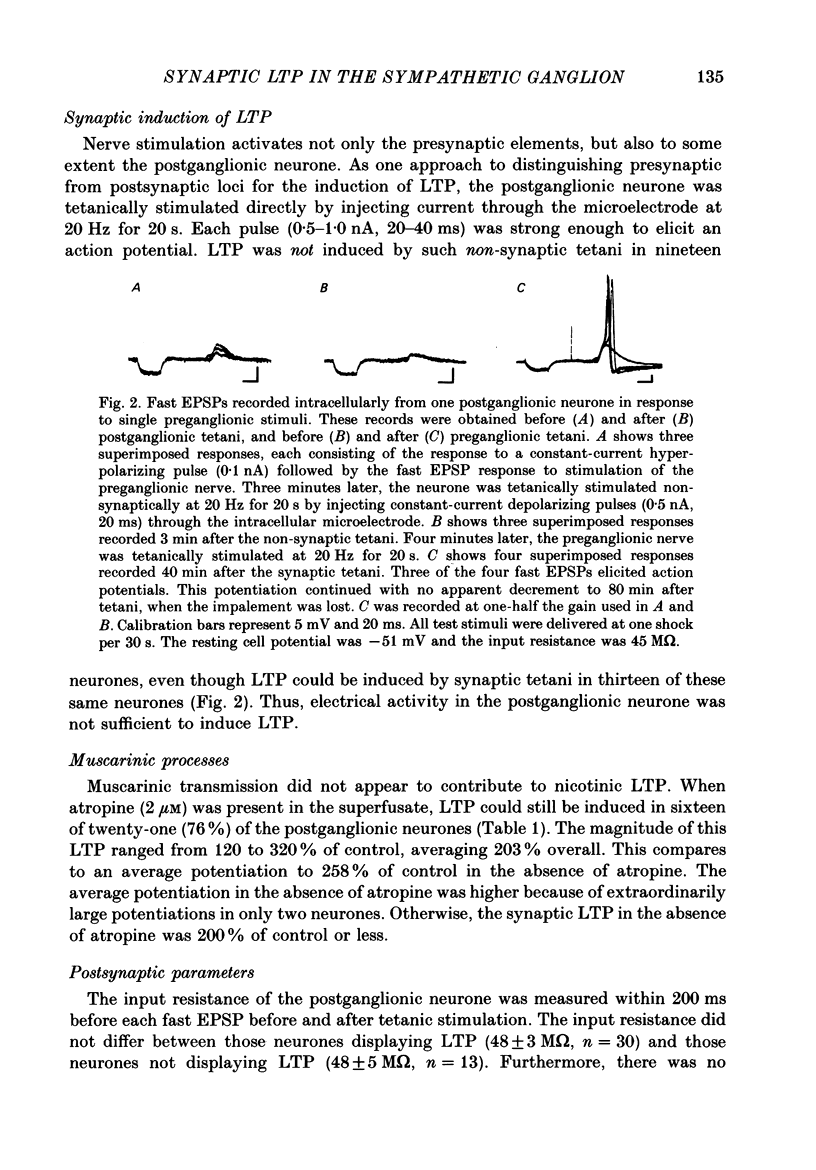
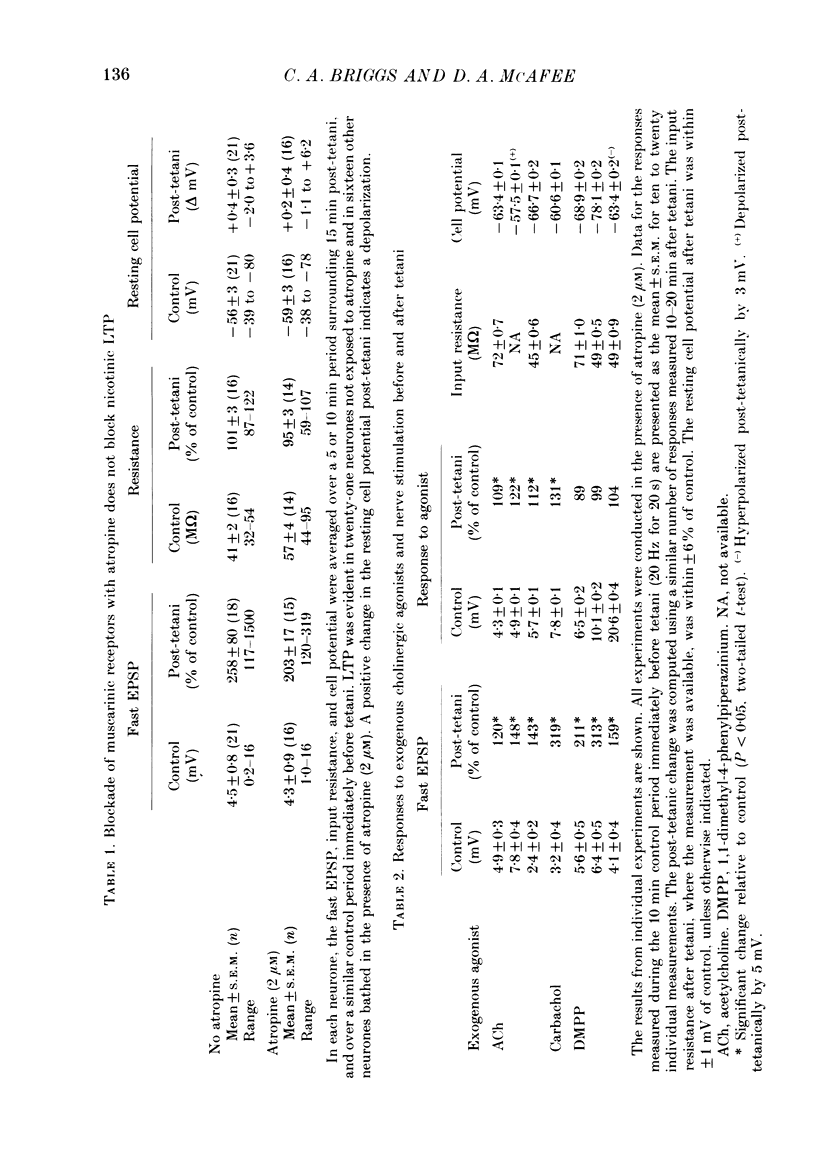
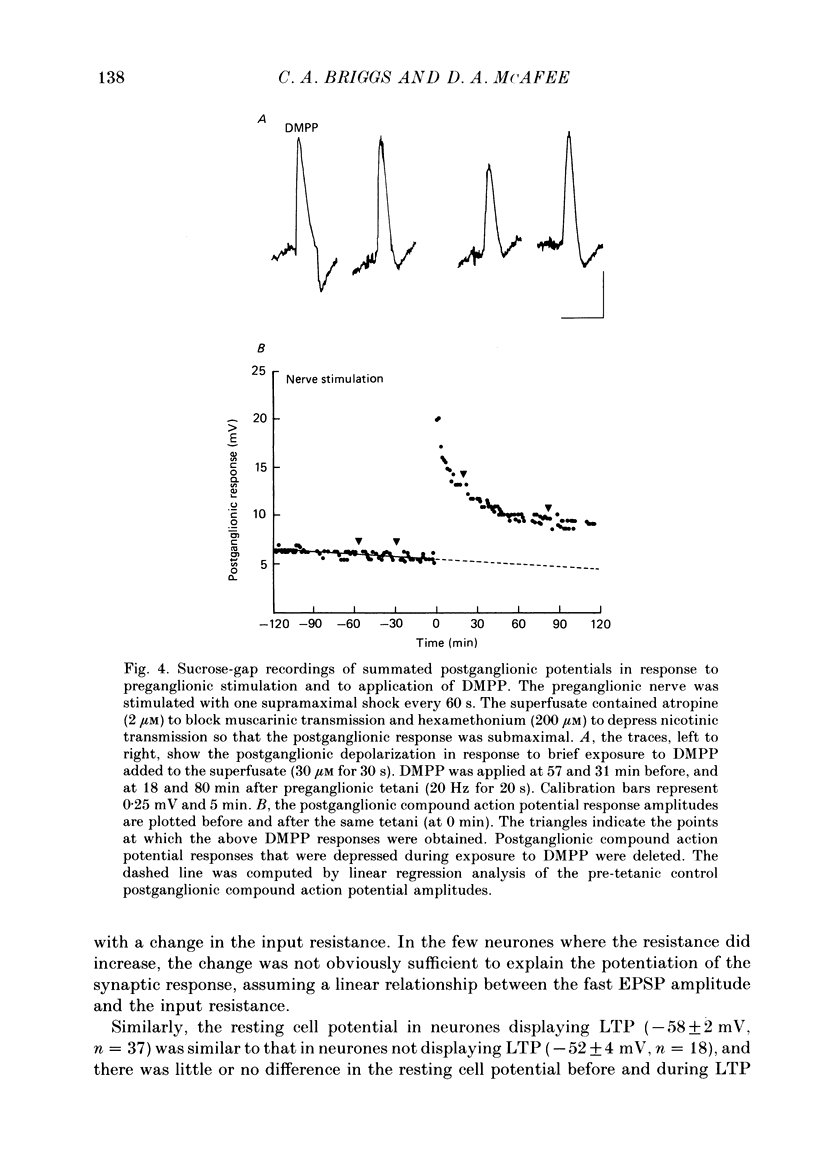
1. Nicotinic fast excitatory postsynaptic potentials (fast EPSPs) were recorded intracellularly from postganglionic neurones in the isolated rat superior cervical ganglion. 2. An hours-long potentiation of the fast EPSP could be induced by brief tetanic stimulation of the preganglionic nerve (5 Hz for 5 s to 20 Hz for 20 s). While long-term potentiation (LTP) can be detected in every ganglion by extracellular techniques, LTP was induced in only two-thirds of the nicotinic synaptic responses. 3. Muscarinic blockade with atropine did not prevent LTP of the fast EPSP. 4. LTP of the fast EPSP did not correlate with changes in input resistance nor cell potential, as recorded in the soma. 5. The formation of nicotinic LTP appeared to depend upon stimulation of the nerve terminals. Non-synaptic tetanic depolarization of the postganglionic neurone, effected by injecting depolarizing current pulses through the intracellular microelectrode, was not sufficient. LTP could be induced by synaptic tetani in two-thirds of the same neurones. 6. The response to exogenous 1,1-dimethyl-4-phenylpiperazinium (DMPP), a selective nicotinic agonist, was not increased during nicotinic synaptic LTP. This was true whether DMPP was applied by pressure-ejection from an extracellular micropipette during intracellular recording, or by brief superfusion during sucrose-gap recording of postganglionic responses. 7. Responses to exogenous acetylcholine and carbachol were increased during nicotinic LTP when these non-selective cholinergic agonists were applied by pressure-ejection during intracellular recording. However, the potentiation of the fast EPSP was always at least twofold greater than the potentiation of the response to these exogenous agonists. 8. Potentiation of the responses to acetylcholine and carbachol may have been due to long-term enhancement of muscarinic responses. Thus, no postsynaptic basis for nicotinic LTP was uncovered in these studies.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate M. D., Kerr D. S., Landfield P. W. Redistribution of synaptic vesicles during long-term potentiation in the hippocampus. Brain Res. 1987 Jan 20;401(2):401–406. doi: 10.1016/0006-8993(87)91429-6. [DOI] [PubMed] [Google Scholar]
- Baxter D. A., Bittner G. D., Brown T. H. Quantal mechanism of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5978–5982. doi: 10.1073/pnas.82.17.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol. 1977 Oct;271(3):847–862. doi: 10.1113/jphysiol.1977.sp012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Douglas R. M., Errington M. L., Lynch M. A. Correlation between long-term potentiation and release of endogenous amino acids from dentate gyrus of anaesthetized rats. J Physiol. 1986 Aug;377:391–408. doi: 10.1113/jphysiol.1986.sp016193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985 Jun;363:181–190. doi: 10.1113/jphysiol.1985.sp015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term regulation of synaptic acetylcholine release and nicotinic transmission: the role of cyclic AMP. Br J Pharmacol. 1988 Feb;93(2):399–411. doi: 10.1111/j.1476-5381.1988.tb11447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. H. Cellular analysis of associative learning. Physiol Rev. 1987 Apr;67(2):329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Collier B., Kwok Y. N., Welner S. A. Increased acetylcholine synthesis and release following presynaptic activity in a sympathetic ganglion. J Neurochem. 1983 Jan;40(1):91–98. doi: 10.1111/j.1471-4159.1983.tb12657.x. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Blockade of ACh potentials by alpha-bungarotoxin in rat superior cervical ganglion cells. Brain Res. 1980 Sep 8;196(2):536–540. doi: 10.1016/0006-8993(80)90421-7. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Feasey K. J., Lynch M. A., Bliss T. V. Long-term potentiation is associated with an increase in calcium-dependent, potassium-stimulated release of [14C]glutamate from hippocampal slices: an ex vivo study in the rat. Brain Res. 1986 Jan 29;364(1):39–44. doi: 10.1016/0006-8993(86)90985-6. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Ho-Asjoe M., Sastry B. R. Tetanic stimulation-induced changes in [3H]glutamate binding and uptake in rat hippocampus. Gen Pharmacol. 1986;17(5):537–542. doi: 10.1016/0306-3623(86)90089-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H., Abraham W. C., Huang Y. Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987 Mar;7(3):774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso S. R., Ganong A. H., Brown T. H. Hebbian synapses in hippocampus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Baudry M., Cummins J. T., Way S., Lynch G. Induction of glutamate binding sites in hippocampal membranes by transient exposure to high concentrations of glutamate or glutamate analogs. J Neurosci. 1986 Feb;6(2):355–363. doi: 10.1523/JNEUROSCI.06-02-00355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Kuba K., Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985 Feb;359:219–233. doi: 10.1113/jphysiol.1985.sp015582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Kumamoto E. Long-term potentiation of transmitter release induced by adrenaline in bull-frog sympathetic ganglia. J Physiol. 1986 May;374:515–530. doi: 10.1113/jphysiol.1986.sp016095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Kobayashi H., Tanaka T. Synaptic coupling into the production and storage of a neuronal memory trace. Nature. 1975 Nov 13;258(5531):155–157. doi: 10.1038/258155a0. [DOI] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch M. A., Errington M. L., Bliss T. V. Long-term potentiation of synaptic transmission in the dentate gyrus: increased release of [14C]glutamate without increase in receptor binding. Neurosci Lett. 1985 Nov 20;62(1):123–129. doi: 10.1016/0304-3940(85)90295-2. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. Changes in statistical release parameters during prolonged stimulation of preganglionic nerve terminals. J Physiol. 1975 Dec;253(2):477–491. doi: 10.1113/jphysiol.1975.sp011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H., Libet B. Stimulation of adenylate cyclase in relation to dopamine-induced long-term enhancement (LTE) of muscarinic depolarization in the rabbit superior cervical ganglion. J Neurosci. 1987 Feb;7(2):311–318. doi: 10.1523/JNEUROSCI.07-02-00311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal mechanism of transmitter release during progressive depletion of the presynaptic stores at a ganglionic synapse. The action of hemicholinium-3 and thiamine deprivation. J Gen Physiol. 1973 Mar;61(3):342–360. doi: 10.1085/jgp.61.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Schulman J. A., Smith P. A., Busis N. A. Long-lasting synaptic potentials and the modulation of synaptic transmission. Fed Proc. 1979 Jun;38(7):2084–2094. [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L., Horn J. P., McAfee D. A., Yarowsky P. J. Facilitation, augmentation, and potentiation of synaptic transmission at the superior cervical ganglion of the rabbit. J Gen Physiol. 1980 Aug;76(2):213–231. doi: 10.1085/jgp.76.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]


