Abstract
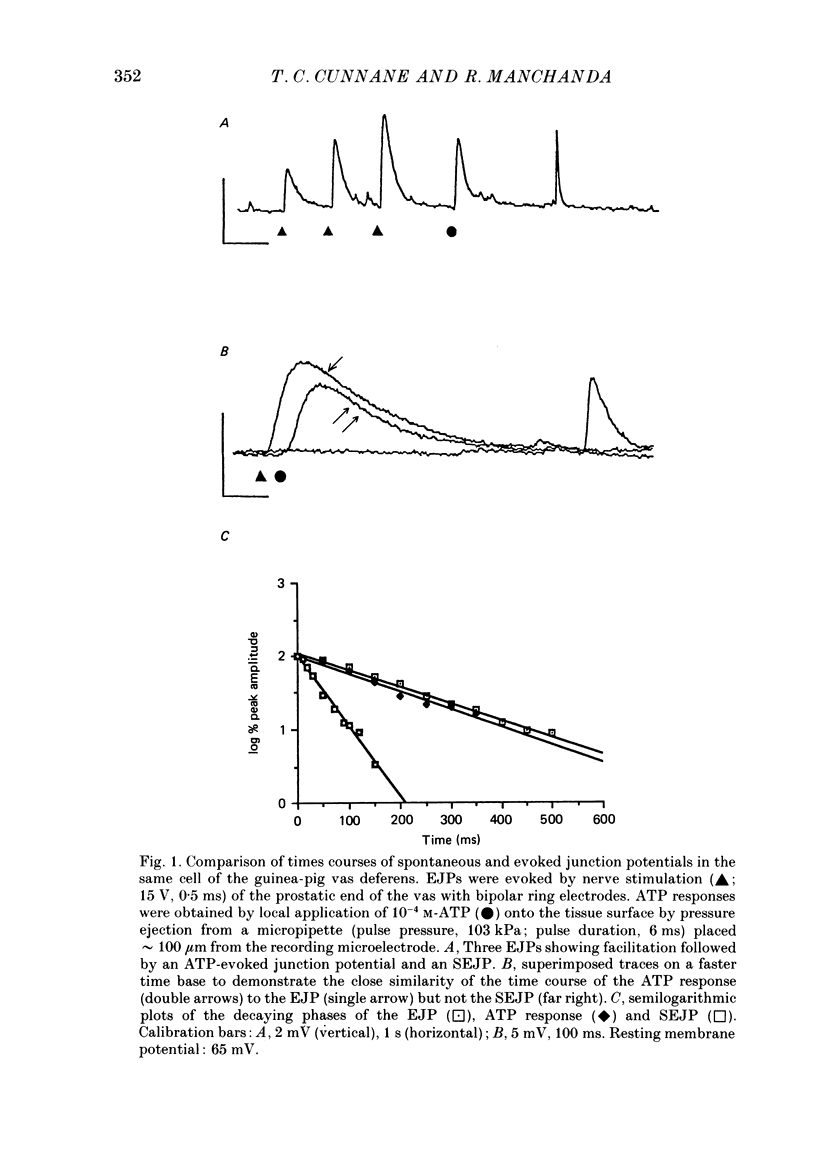
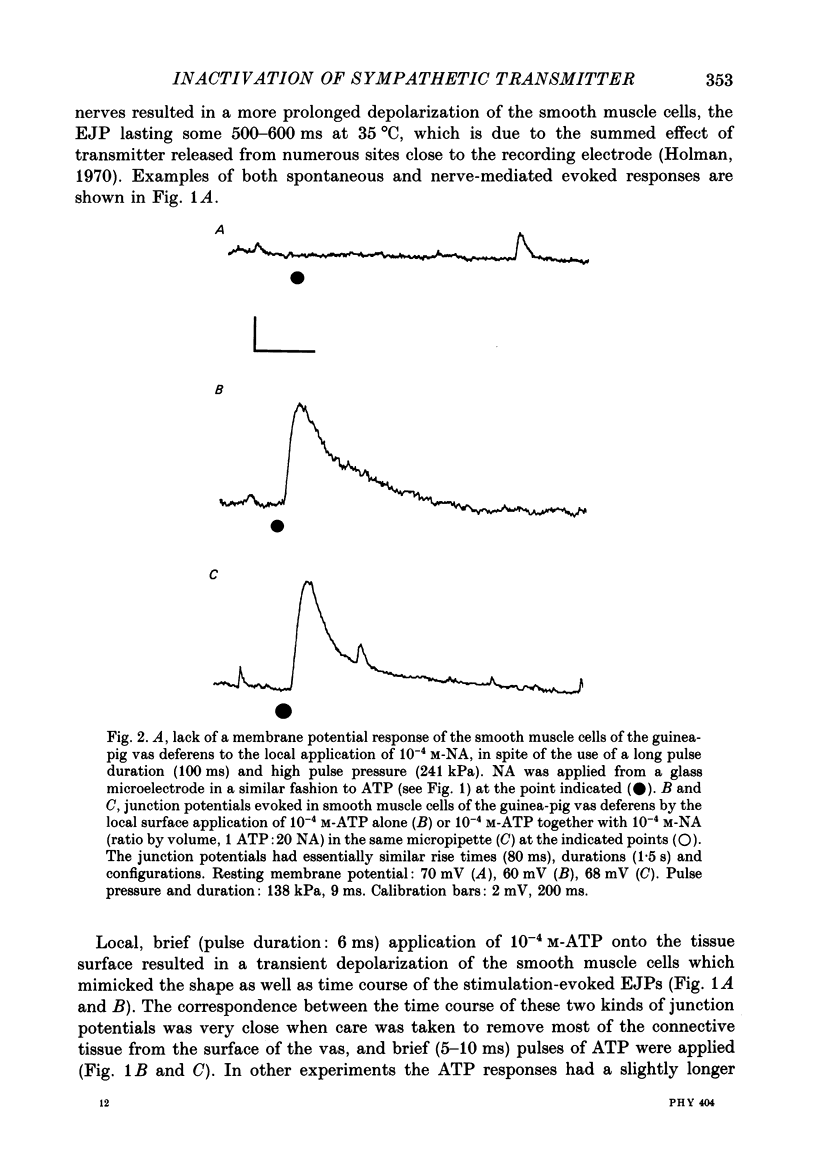
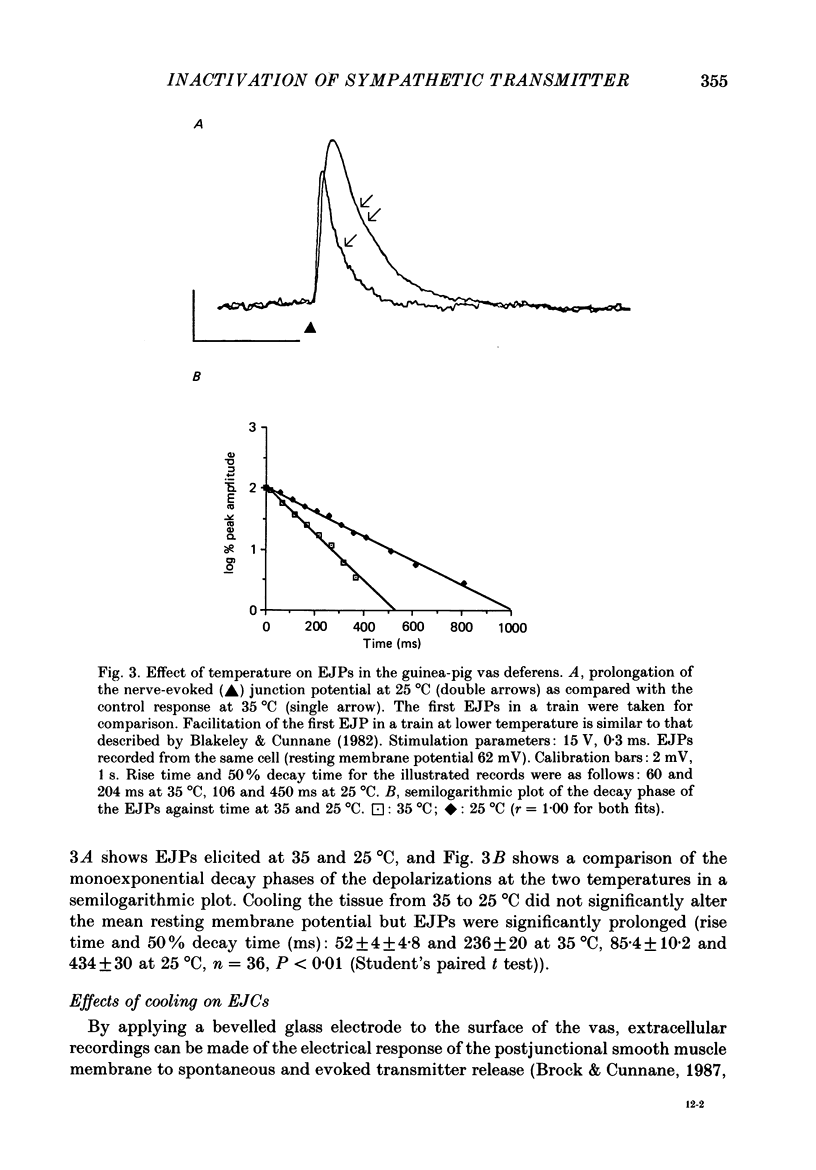
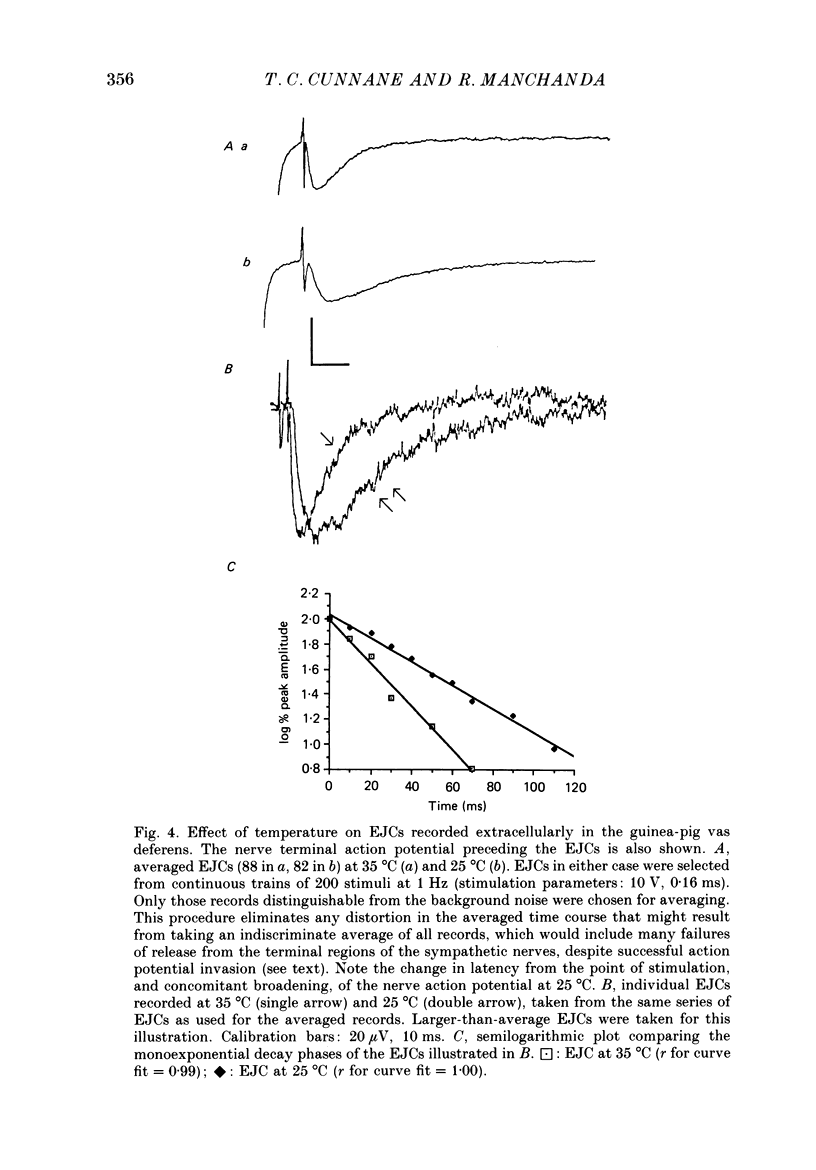
1. The properties of junction potentials evoked by nerve stimulation and by local application of drugs, and currents evoked by nerve stimulation, in the smooth muscle cells of the guinea-pig vas deferens have been investigated. The effects of temperature on these responses have been studied using intracellular and extracellular recording. 2. Local, brief (5-15 ms) application of 10(-4) M-adenosine-5'-triphosphate (ATP) from glass micropipettes onto the surface of the vas deferens, using pressure pulses (103-206 kPa), elicited a depolarization of the smooth muscle cell membranes which closely resembled the nerve stimulation-evoked excitatory junction potential (EJP). 3. Local application of 10(-4) M-noradrenaline (NA) failed to produce any detectable membrane potential response. Junction potentials elicited by a mixture of 10(-4) M-ATP and 10(-4) M-NA (ratio by volume 1:50) in the drug ejection micropipette were similar in shape to those evoked by ATP alone. 4. Cooling the tissue from 35 to 25 degrees C did not significantly alter resting membrane potentials but resulted in a significant prolongation of the rising and decaying phases of the EJPs. Fifty per cent decay times for EJPs at 35 and 25 degrees C were (mean +/- S.D.) 236 +/- 20 and 434 +/- 30 ms respectively (P less than 0.01). 5. Extracellularly recorded excitatory junction currents (EJCs) elicited by nerve stimulation, believed to reflect the transmembrane current underlying the EJPs, were prolonged in parallel at low temperatures (50% decay times of EJCs at 35 and 25 degrees C: 11.73 +/- 3.94 and 26.15 +/- 8.4 ms, respectively, P less than 0.01). 6. Junction potentials evoked by locally applied, exogenous ATP were also significantly prolonged by cooling (50% decay times: 663 +/- 88 ms at 35 degrees C and 1955 +/- 79 ms at 25 degrees C, P less than 0.01). 7. Bath application of 10(-6) M-alpha,beta-methylene ATP, the enzymatically stable, desensitizing analogue of ATP, reversibly abolished nerve-evoked EJPs. Local application of 10(-6) M-alpha,beta-methylene ATP led to a prolonged depolarization of the smooth muscle cells lasting between 20 and 60 s. 8. Junction potentials elicited by locally applied alpha,beta-methylene ATP were not prolonged or otherwise significantly altered on cooling. The durations of the depolarizations were 46.0 +/- 12.1 s at 35 degrees C and 43.4 +/- 10.6 s at 25 degrees C (P greater than 0.1).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




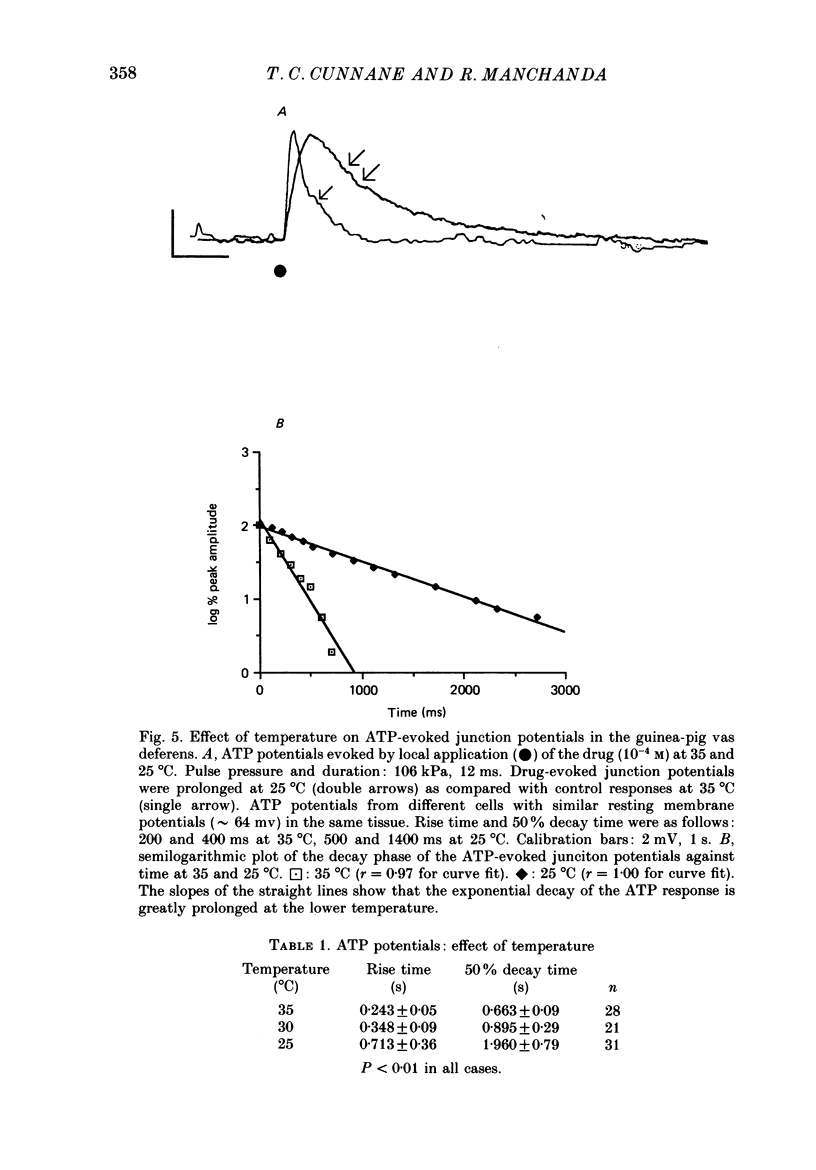




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcorn R. J., Cunnane T. C., Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986 Dec;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
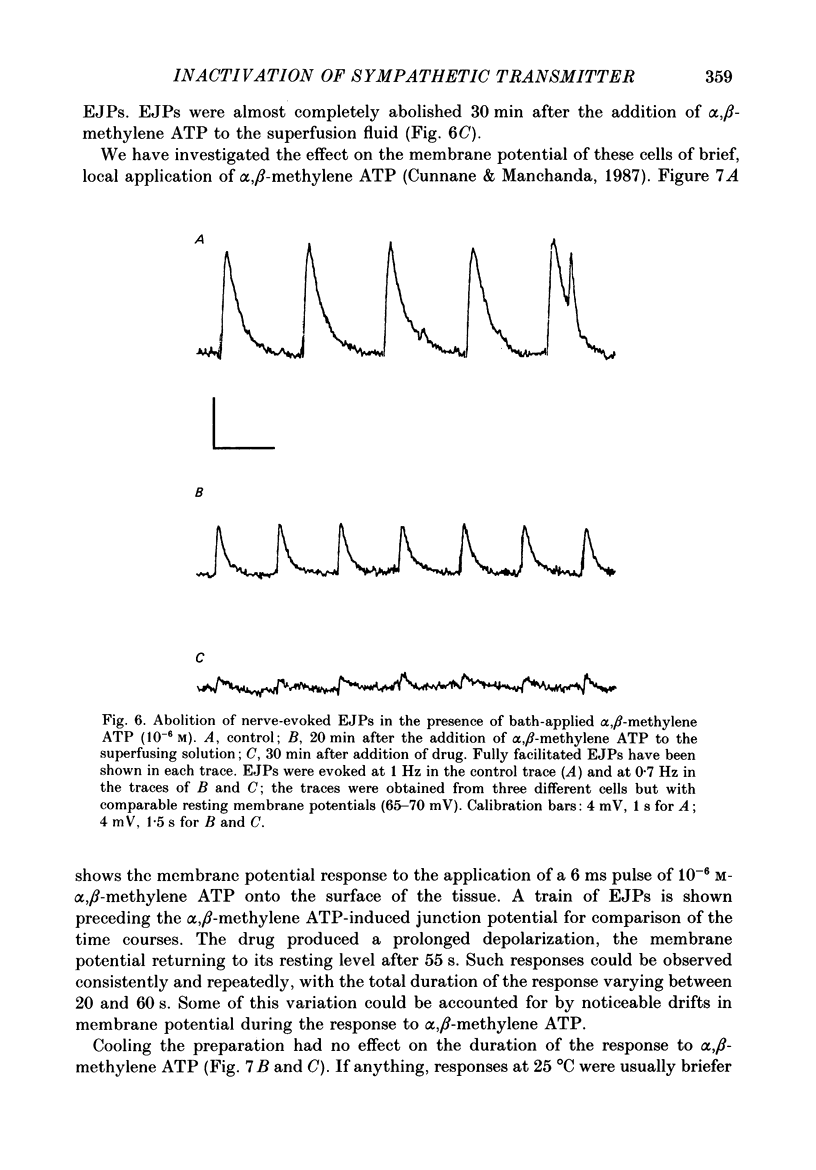
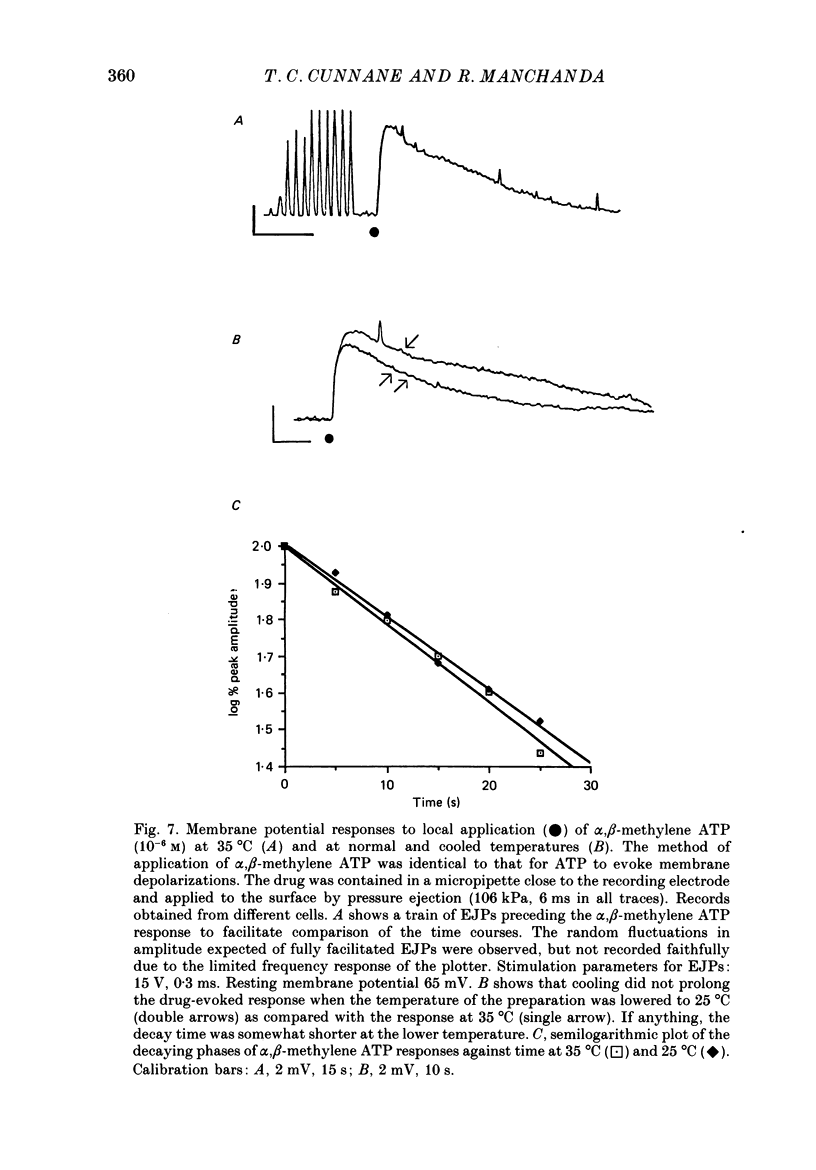
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrand P., Brock J. A., Cunnane T. C. Time course of transmitter action at the sympathetic neuroeffector junction in rodent vascular and non-vascular smooth muscle. J Physiol. 1988 Jul;401:657–670. doi: 10.1113/jphysiol.1988.sp017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Spontaneous potential at sympathetic nerve endings in smooth muscle. J Physiol. 1962 Mar;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Cunnane T. C. An electrophysiological analysis of the effects of cooling on autonomic neuromuscular transmission in the guinea-pig vas deferens. Q J Exp Physiol. 1982 Oct;67(4):617–628. doi: 10.1113/expphysiol.1982.sp002681. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Electrical activity at the sympathetic neuroeffector junction in the guinea-pig vas deferens. J Physiol. 1988 May;399:607–632. doi: 10.1113/jphysiol.1988.sp017099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987 Apr 9;326(6113):605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986 Jan;126(1):67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane T. C., Stjärne L. Secretion of transmitter from individual varicosities of guinea-pig and mouse vas deferens: all-or-none and extremely intermittent. Neuroscience. 1982;7(11):2565–2576. doi: 10.1016/0306-4522(82)90085-9. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Fried G., Hedqvist P. Origin of adenosine released from rat vas deferens by nerve stimulation. Eur J Pharmacol. 1982 Apr 23;79(3-4):233–243. doi: 10.1016/0014-2999(82)90629-x. [DOI] [PubMed] [Google Scholar]
- Hamlyn J. M., Senior A. E. Evidence that Mg2+- or Ca2+-activated adenosine triphosphatase in rat pancreas is a plasma-membrane ecto-enzyme. Biochem J. 1983 Jul 15;214(1):59–68. doi: 10.1042/bj2140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. THE EFFECT OF TEMPERATURE ON NEUROMUSCULAR TRANSMISSION IN THE VAS DEFERENS OF THE GUINEA-PIG. J Physiol. 1964 Apr;170:561–570. doi: 10.1113/jphysiol.1964.sp007349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew M. J., White T. D. Release of endogenous ATP during sympathetic nerve stimulation. Br J Pharmacol. 1987 Oct;92(2):349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. C. Adrenergic and 'non-adrenergic' components in the contractile response of the vas deferens to a single indirect stimulus. J Physiol. 1978 Oct;283:23–39. doi: 10.1113/jphysiol.1978.sp012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Williams J. T. Inhibitory synaptic potentials recorded from mammalian neurones prolonged by blockade of noradrenaline uptake. J Physiol. 1987 Jan;382:87–103. doi: 10.1113/jphysiol.1987.sp016357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Electrical responses of smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J Physiol. 1985 Feb;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall D. P., Stitzel R. E., Rowe J. N. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978 Jul 1;50(1):27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]


