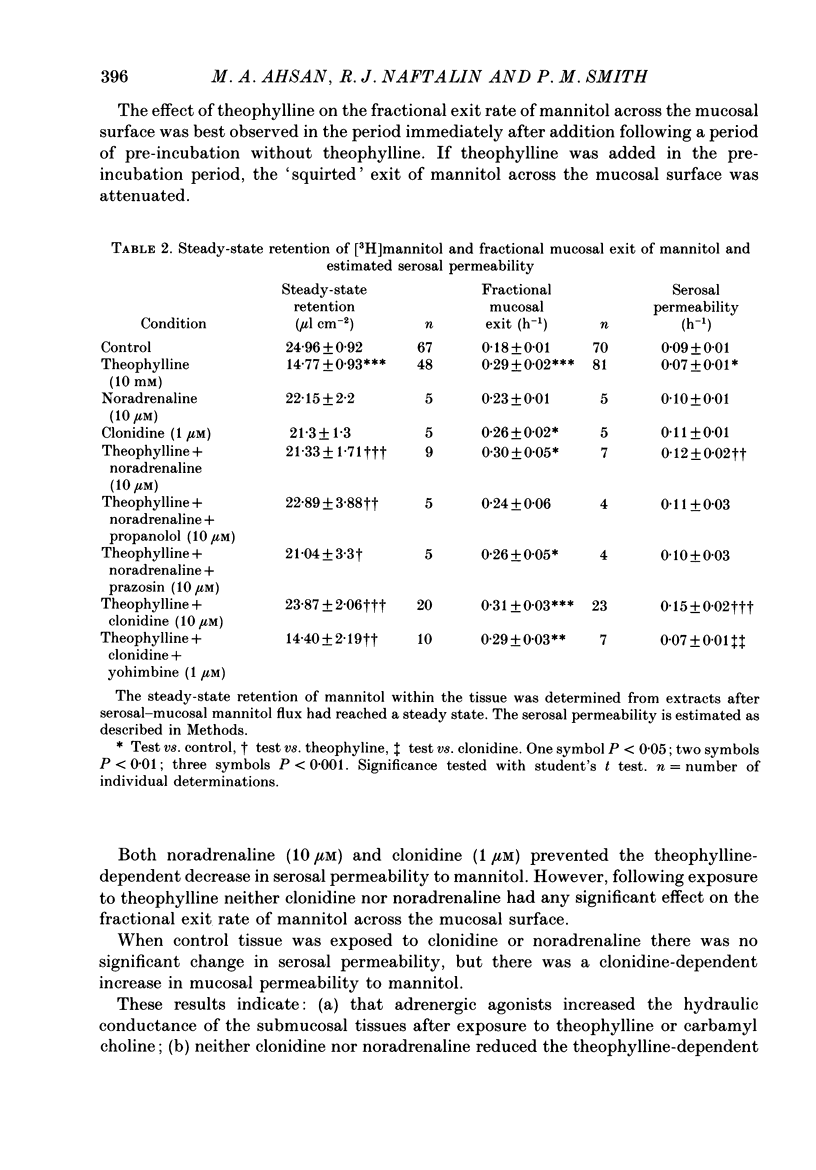
Abstract
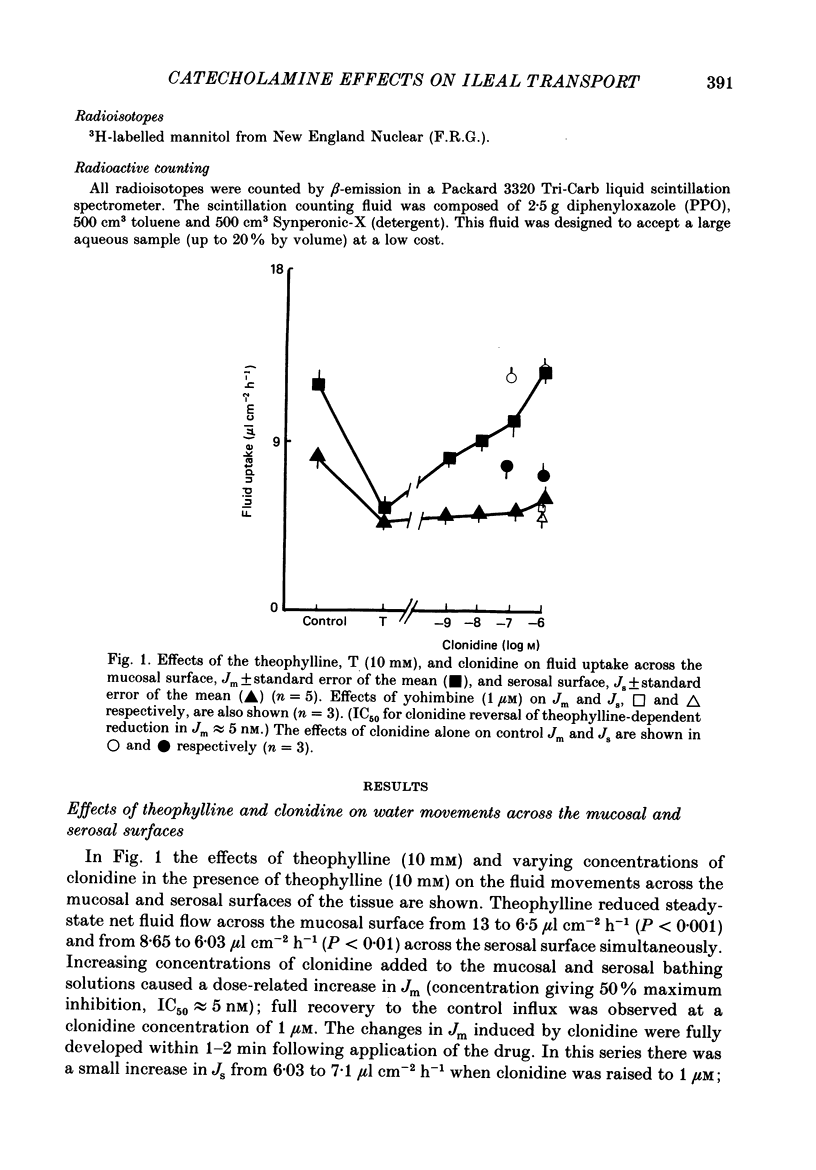
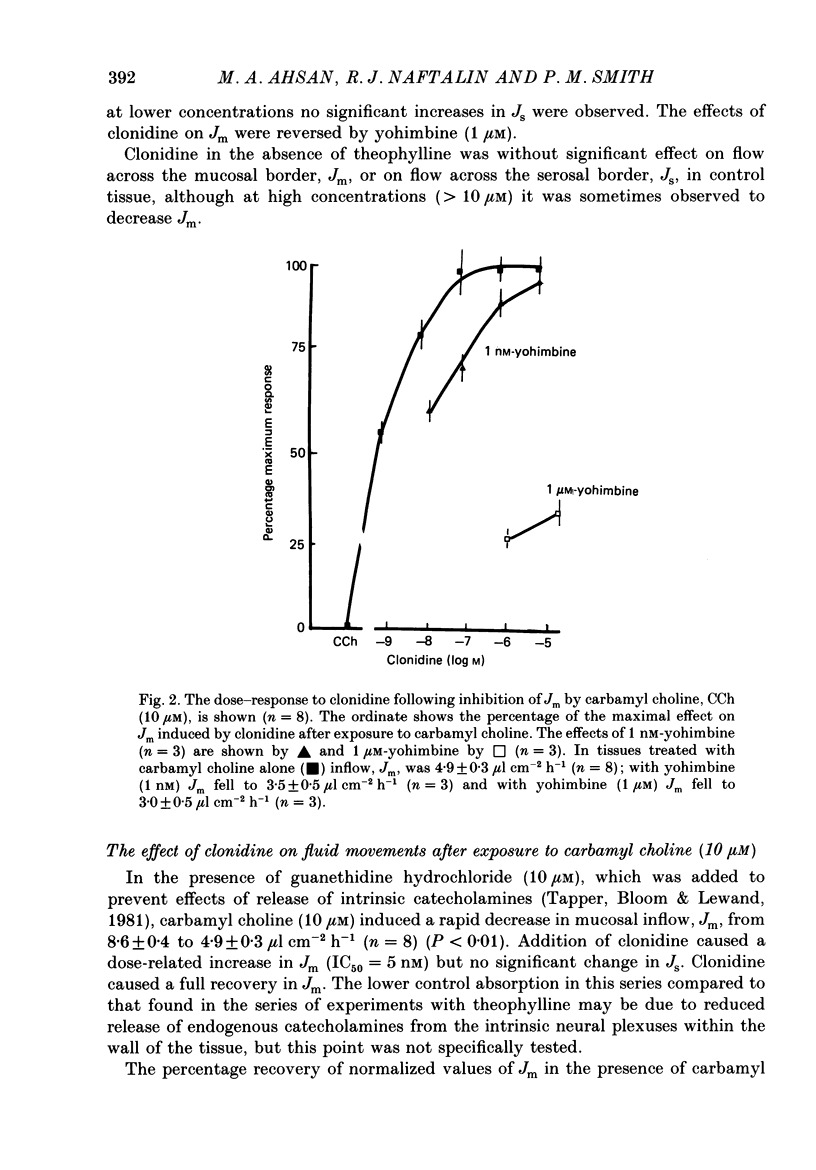
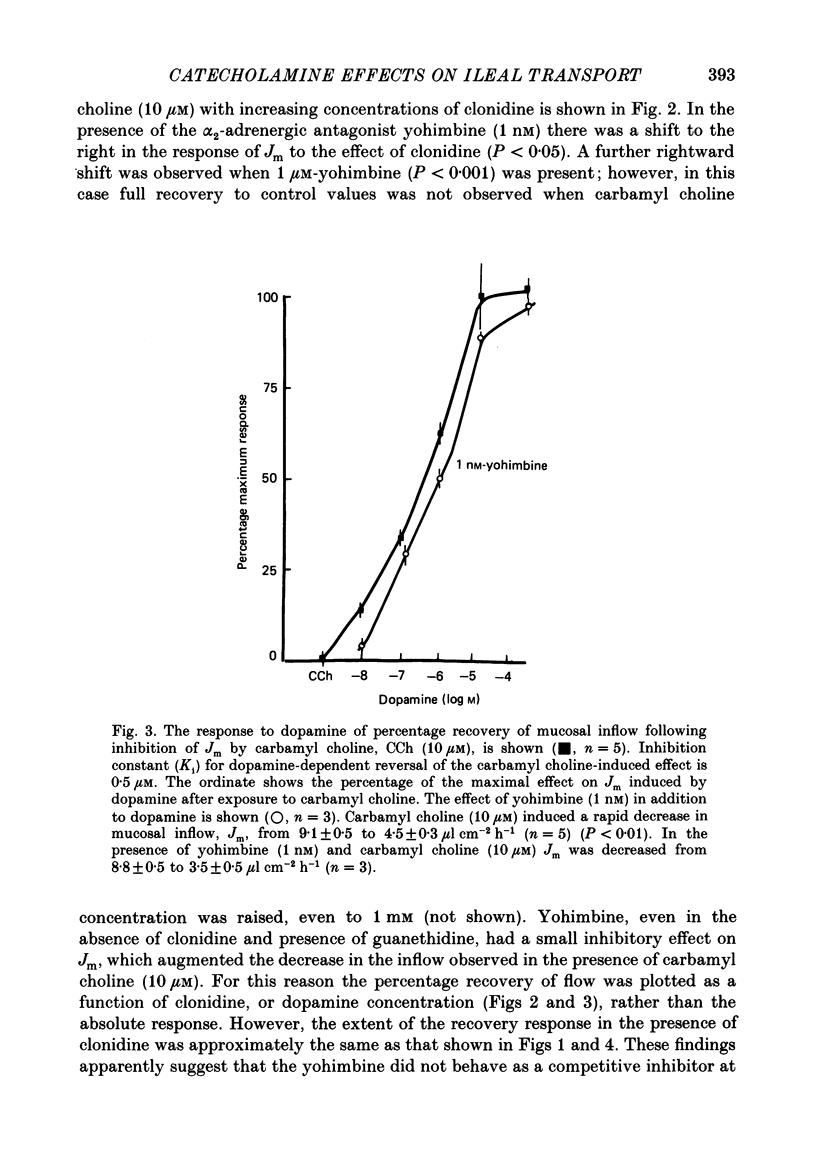
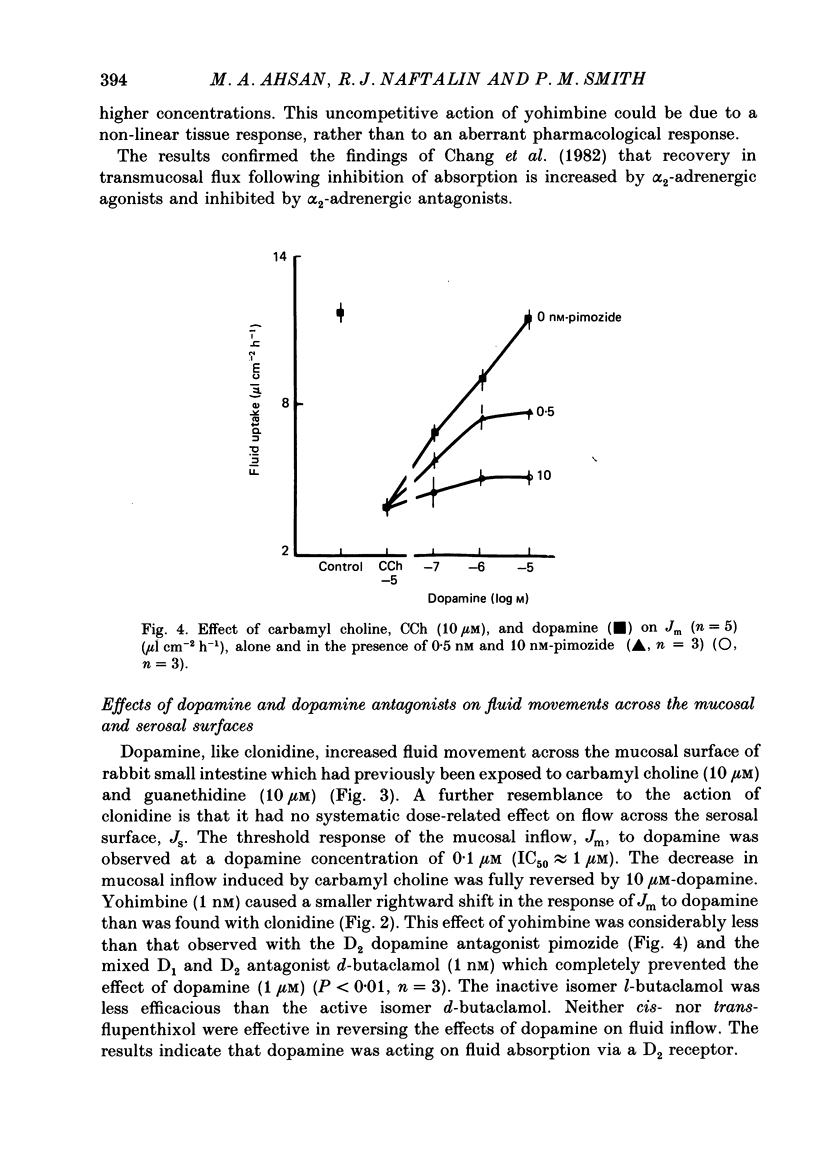
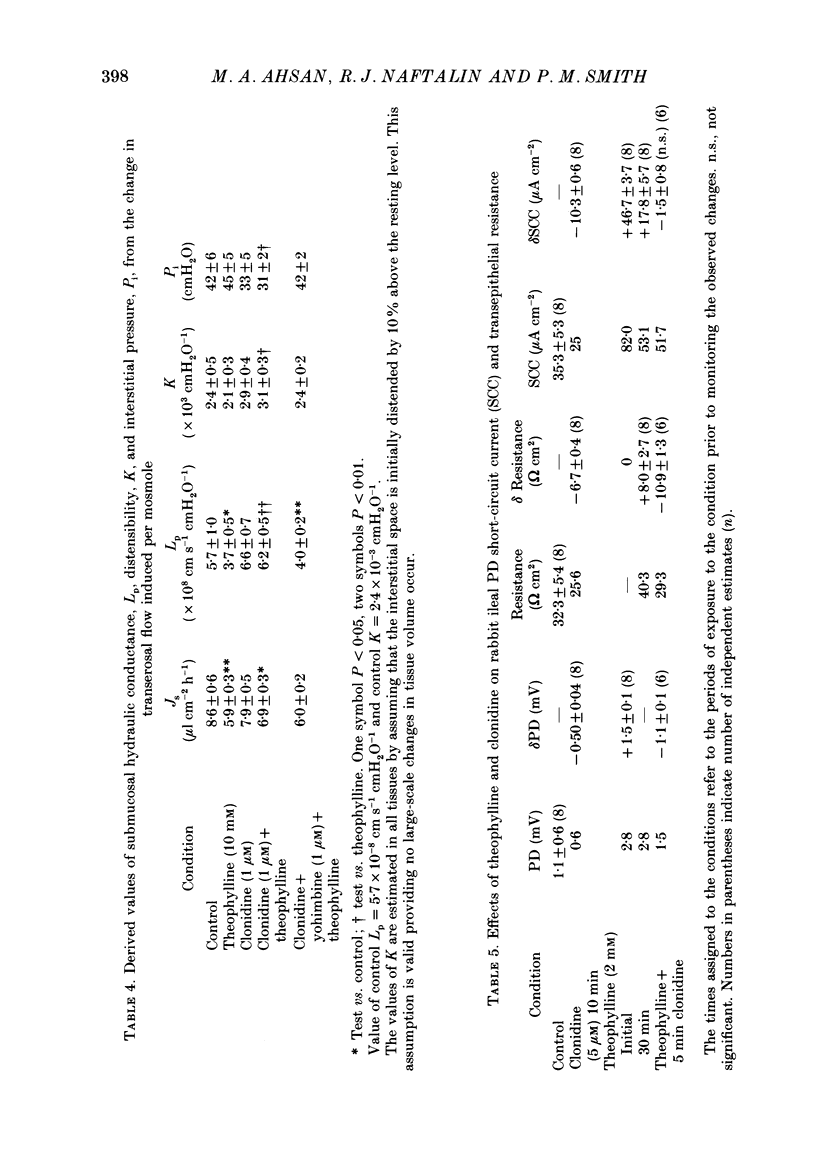
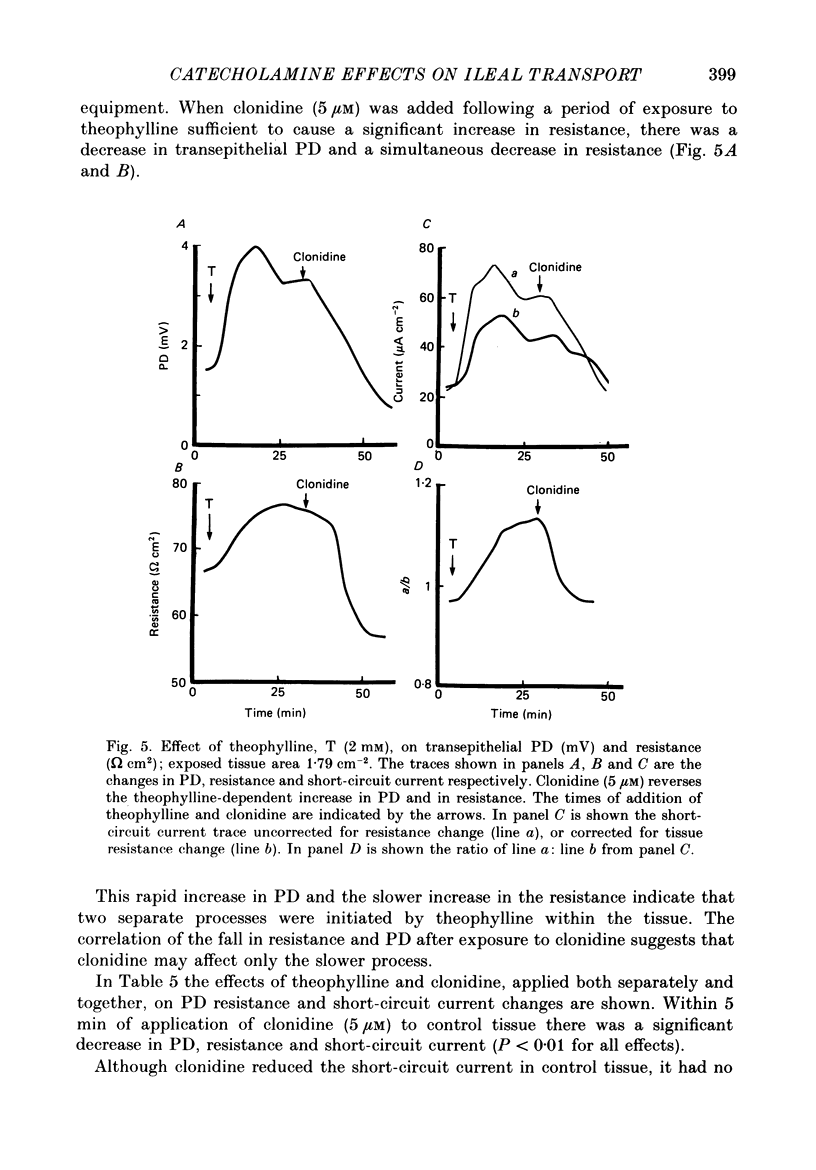
1. The effects of clonidine and dopamine on water movements across the mucosal and serosal surfaces of rabbit ileum have been investigated using a high-resolution method for monitoring water flows in vitro. 2. Theophylline (10 mM) and carbamyl choline (10 microM) caused a reduction in fluid inflow across the mucosal surface and a smaller decrease in fluid outflow across the serosal surface. Addition of the alpha 2-adrenergic agonist clonidine or dopamine fully reversed the theophylline, or carbamyl choline-induced decrease in mucosal inflow in a dose-related manner. 3. The effects of clonidine on mucosal inflow are blocked by the alpha 2-adrenergic antagonist, yohimbine. Yohimbine was much less effective than pimozide or d-butaclamol in blocking the effect of dopamine on mucosal inflow. These findings support the view that there are separate alpha 2-adrenergic and dopaminergic receptors. 4. The hydraulic conductance (Lp) of the serosal surface was measured directly from the change in serosal exit flow following addition of 2 mosmol kg-1 of polyethylene glycol (molecular mass 20,000 Da) to the serosal bathing solution. Theophylline reduced the Lp by 35%. Clonidine (1 microM) added to theophylline-treated tissues increased the Lp by 66%. This effect was prevented by yohimbine (1 microM). 5. The effects of theophylline, clonidine and dopamine on the permeability of the mucosal and serosal surfaces of the tissue to [3H]mannitol were measured. These showed that theophylline increased the rate of labelled mannitol loss across the mucosal surface but reduced the mannitol permeability across the serosal surface. This latter effect was reversed by clonidine and dopamine. 6. Changes in transepithelial electrical potential difference (PD), short-circuit current and resistance were monitored. Theophylline caused a rapid increase in PD and short-circuit current and a slower increase in resistance. Clonidine (5 microM) reversed the effects on PD and resistance but was without significant effect on short-circuit current. The results suggest that a major component of secretagogue-induced reduction in fluid transport in vitro is due to mechanical changes in the submucosa, probably induced by modulation of neurotransmitter release within the tissue.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahsan M. A., Ilundain A., Naftalin R. J., Sandhu B. K., Smith P. M. Effects of theophylline, choleragen and loperamide on rabbit ileal fluid and electrolyte transport in vitro. Br J Pharmacol. 1987 Dec;92(4):743–754. doi: 10.1111/j.1476-5381.1987.tb11378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Aceves J., Mandel L. J. Potassium uptake across serosal surface of isolated frog skin epithelium. Am J Physiol. 1972 Jun;222(6):1366–1373. doi: 10.1152/ajplegacy.1972.222.6.1366. [DOI] [PubMed] [Google Scholar]
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Field M., Miller R. J. alpha 2-Adrenergic receptor regulation of ion transport in rabbit ileum. Am J Physiol. 1982 Mar;242(3):G237–G242. doi: 10.1152/ajpgi.1982.242.3.G237. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cusolito S., Battisti L., Fogel R., Sharp G. W. Dopamine stimulation of active Na and Cl absorption in rabbit ileum: interaction with alpha 2-adrenergic and specific dopamine receptors. J Clin Invest. 1982 Apr;69(4):1008–1016. doi: 10.1172/JCI110504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Elta G., Battisti L., Fogel R., Label-Schwartz E. Effect of dopamine and bromocriptine on rat ileal and colonic transport. Stimulation of absorption and reversal of cholera toxin-induced secretion. Gastroenterology. 1983 Mar;84(3):516–523. [PubMed] [Google Scholar]
- Drew G. M. Pharmacological characterization of the presynaptic alpha-adrenoceptors regulating cholinergic activity in the guinea-pig ileum. Br J Pharmacol. 1978 Oct;64(2):293–300. doi: 10.1111/j.1476-5381.1978.tb17303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- Field M., Sheerin H. E., Henderson A., Smith P. L. Catecholamine effects on cyclic AMP levels and ion secretion in rabbit ileal mucosa. Am J Physiol. 1975 Jul;229(1):86–92. doi: 10.1152/ajplegacy.1975.229.1.86. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Fromm M., Schulzke J. D., Hegel U. Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch. 1985 Dec;405(4):400–402. doi: 10.1007/BF00595695. [DOI] [PubMed] [Google Scholar]
- Görich R., Weihrauch T. R., Kilbinger H. The inhibition by dopamine of cholinergic transmission in the isolated guinea-pig ileum. Mediation through alpha-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):308–312. doi: 10.1007/BF00501170. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Naftalin R. J., Simmons N. L., Walker M. Electrophysiological and electron-microscopical correlations with fluid and electrolyte secretion in rabbit ileum. J Physiol. 1979 May;290(2):367–386. doi: 10.1113/jphysiol.1979.sp012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal ion transport: effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976 Jul;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- Johnsen J. A., Levine M. W. Correlation of activity in neighbouring goldfish ganglion cells: relationship between latency and lag. J Physiol. 1983 Dec;345:439–449. doi: 10.1113/jphysiol.1983.sp014987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Increase by alpha-adrenolytic drugs of acetylcholine release evoked by field stimulation of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(3):255–257. doi: 10.1007/BF00504758. [DOI] [PubMed] [Google Scholar]
- Lee J. S. Tissue fluid pressure, lymph pressure, and fluid transport in rat intestinal villi. Microvasc Res. 1986 Mar;31(2):170–183. doi: 10.1016/0026-2862(86)90032-4. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Holman G. D. The role of Na as a determinant of the asymmetric permeability of rabbit ileal brush-border to D-galactose. Biochim Biophys Acta. 1974 Dec 24;373(3):453–470. doi: 10.1016/0005-2736(74)90025-x. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Simmons N. L. The effects of theophylline and choleragen on sodium and chloride ion movements within isolated rabbit ileum. J Physiol. 1979 May;290(2):331–350. doi: 10.1113/jphysiol.1979.sp012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J Physiol. 1985 Mar;360:27–50. doi: 10.1113/jphysiol.1985.sp015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. The roles of paracellular and transcellular pathways and submucosal space in isotonic water absorption by rabbit ileum. J Physiol. 1986 Jan;370:409–432. doi: 10.1113/jphysiol.1986.sp015942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaki T., Nakadate T., Yamamoto S., Kato R. Alpha 2-adrenergic receptor in intestinal epithelial cells. Identification by [3H]yohimbine and failure to inhibit cyclic AMP accumulation. Mol Pharmacol. 1983 Jan;23(1):228–234. [PubMed] [Google Scholar]
- Nakaki T., Nakadate T., Yamamoto S., Kato R. Alpha 2-adrenoceptors inhibit the cholera-toxin-induced intestinal fluid accumulation. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):181–184. doi: 10.1007/BF00500478. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Nakadate T., Yamamoto S., Kato R. Alpha-2 adrenergic inhibition of intestinal secretion induced by prostaglandin E1, vasoactive intestinal peptide and dibutyryl cyclic AMP in rat jejunum. J Pharmacol Exp Ther. 1982 Mar;220(3):637–641. [PubMed] [Google Scholar]
- PARSONS D. S., WINGATE D. L. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochim Biophys Acta. 1961 Jan 1;46:170–183. doi: 10.1016/0006-3002(61)90660-6. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. U., Reuss L. Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1983 May;81(5):705–729. doi: 10.1085/jgp.81.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMYTH D. H., TAYLOR C. B. Transfer of water and solutes by an in vitro intestinal preparation. J Physiol. 1957 May 23;136(3):632–648. doi: 10.1113/jphysiol.1957.sp005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjövall H., Redfors S., Hallbäck D. A., Eklund S., Jodal M., Lundgren O. The effect of splanchnic nerve stimulation on blood flow distribution, villous tissue osmolality and fluid and electrolyte transport in the small intestine of the cat. Acta Physiol Scand. 1983 Mar;117(3):359–365. doi: 10.1111/j.1748-1716.1983.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Tapper E. J., Bloom A. S., Lewand D. L. Endogenous norepinephrine release induced by tyramine modulates intestinal ion transport. Am J Physiol. 1981 Sep;241(3):G264–G269. doi: 10.1152/ajpgi.1981.241.3.G264. [DOI] [PubMed] [Google Scholar]
- Tapper E. J., Powell D. W., Morris S. M. Cholinergic-adrenergic interactions on intestinal ion transport. Am J Physiol. 1978 Oct;235(4):E402–E409. doi: 10.1152/ajpendo.1978.235.4.E402. [DOI] [PubMed] [Google Scholar]
- Wahawisan R., Gaginella T. S., Wallace L. J. Jejunal-ileal differences in dopaminergic but not alpha-adrenergic antisecretory effects. Am J Physiol. 1985 Mar;248(3 Pt 1):G332–G336. doi: 10.1152/ajpgi.1985.248.3.G332. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Wiedner G., Wright E. M. Volume flows across gallbladder epithelium induced by small hydrostatic and osmotic gradients. J Membr Biol. 1979 Aug;49(1):1–20. doi: 10.1007/BF01871037. [DOI] [PubMed] [Google Scholar]


