Abstract
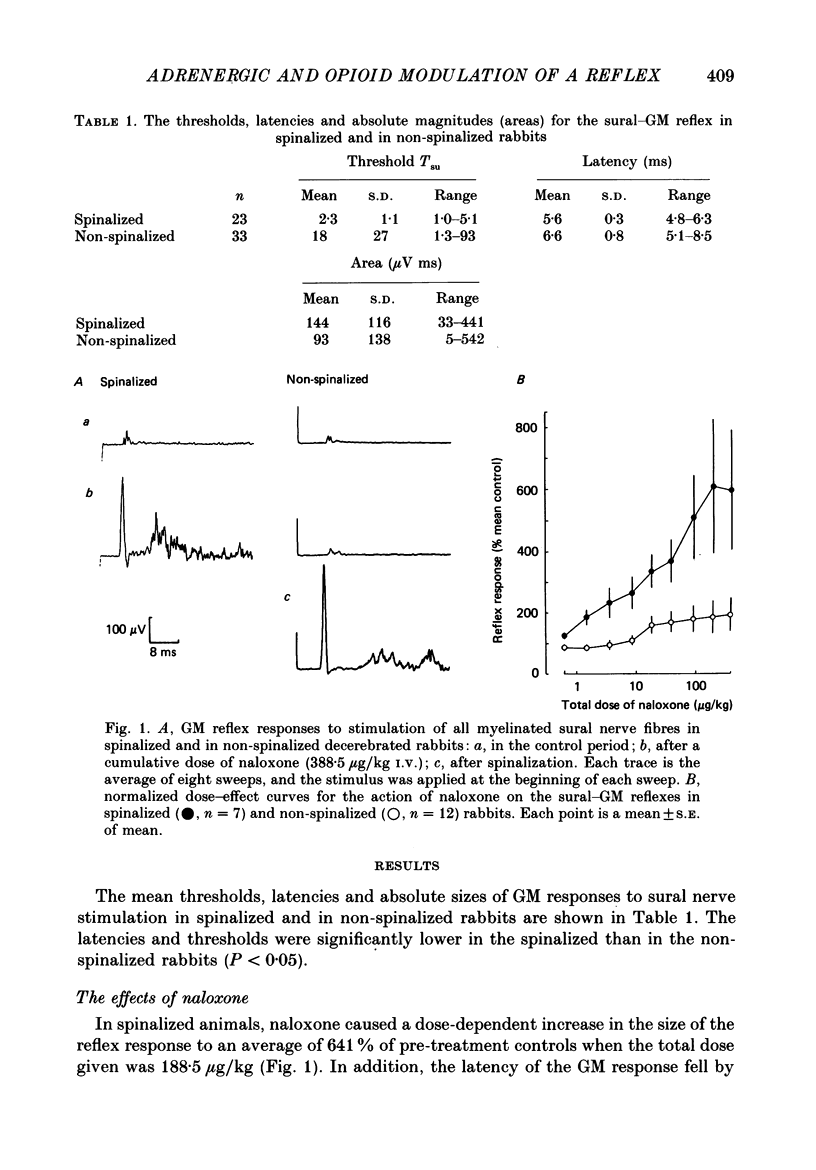
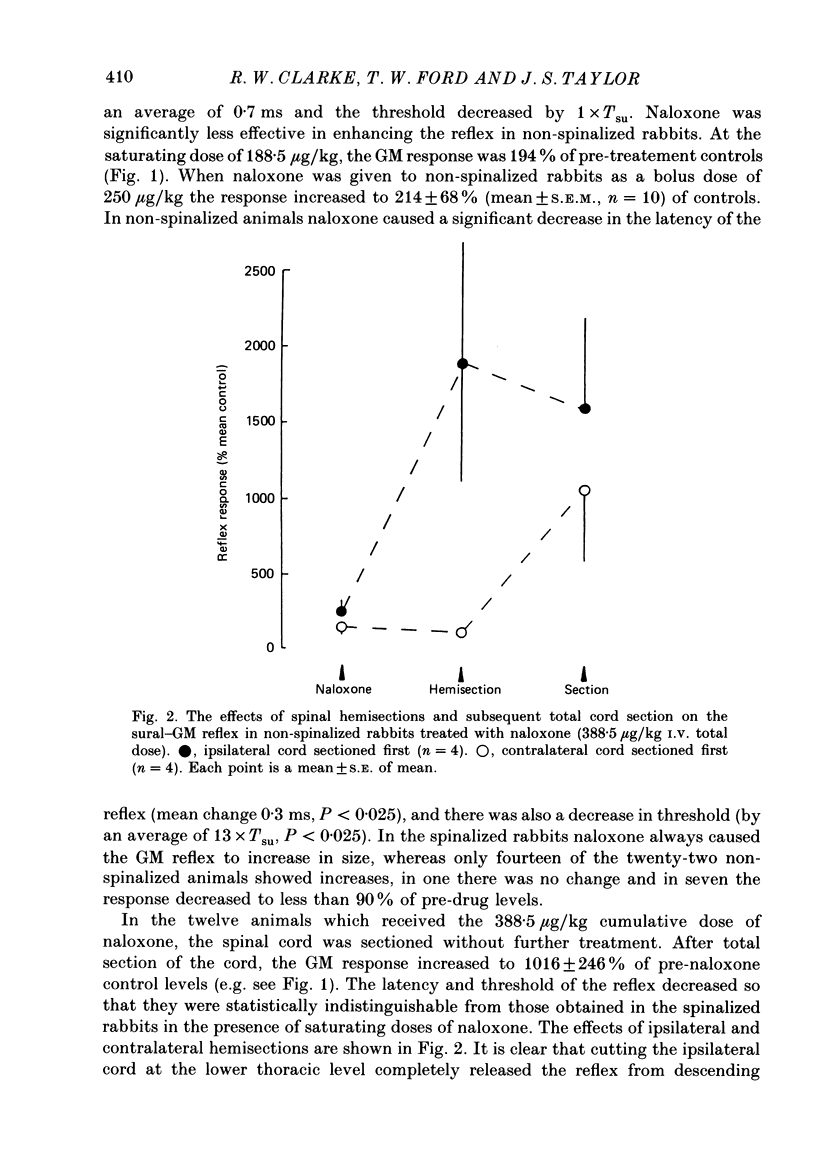
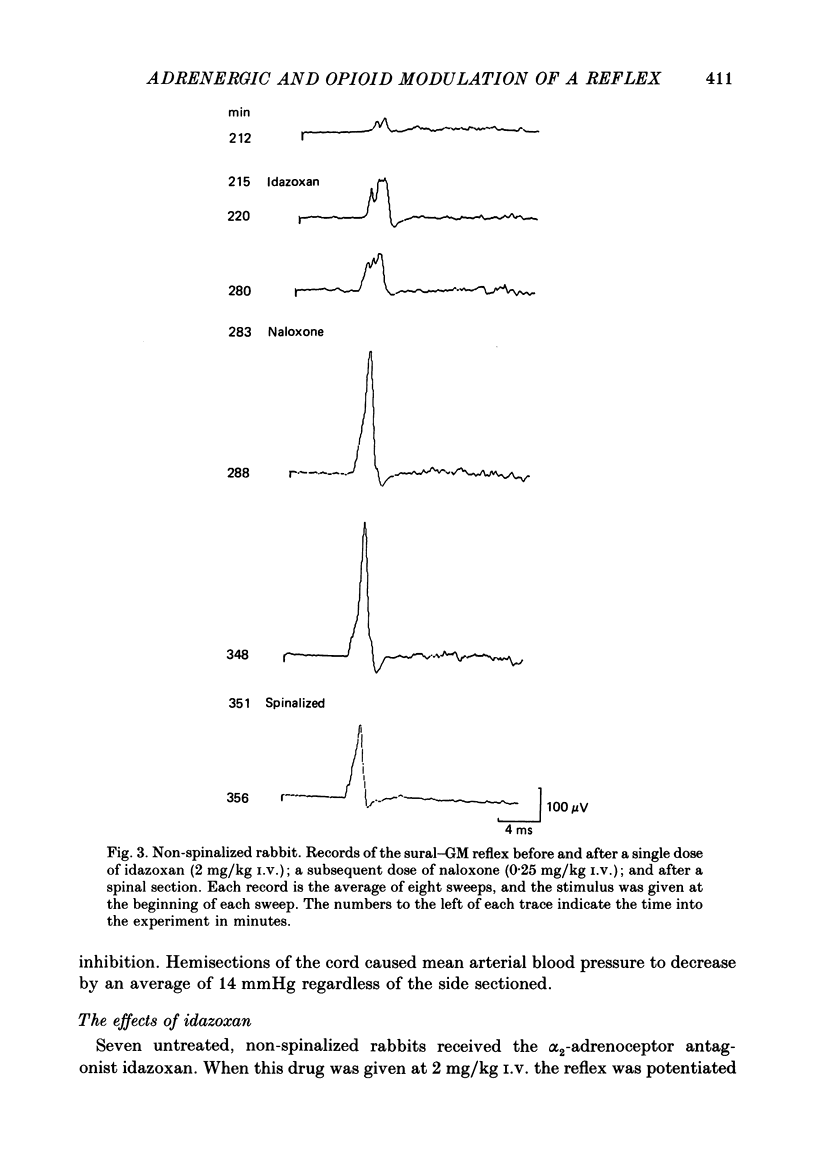
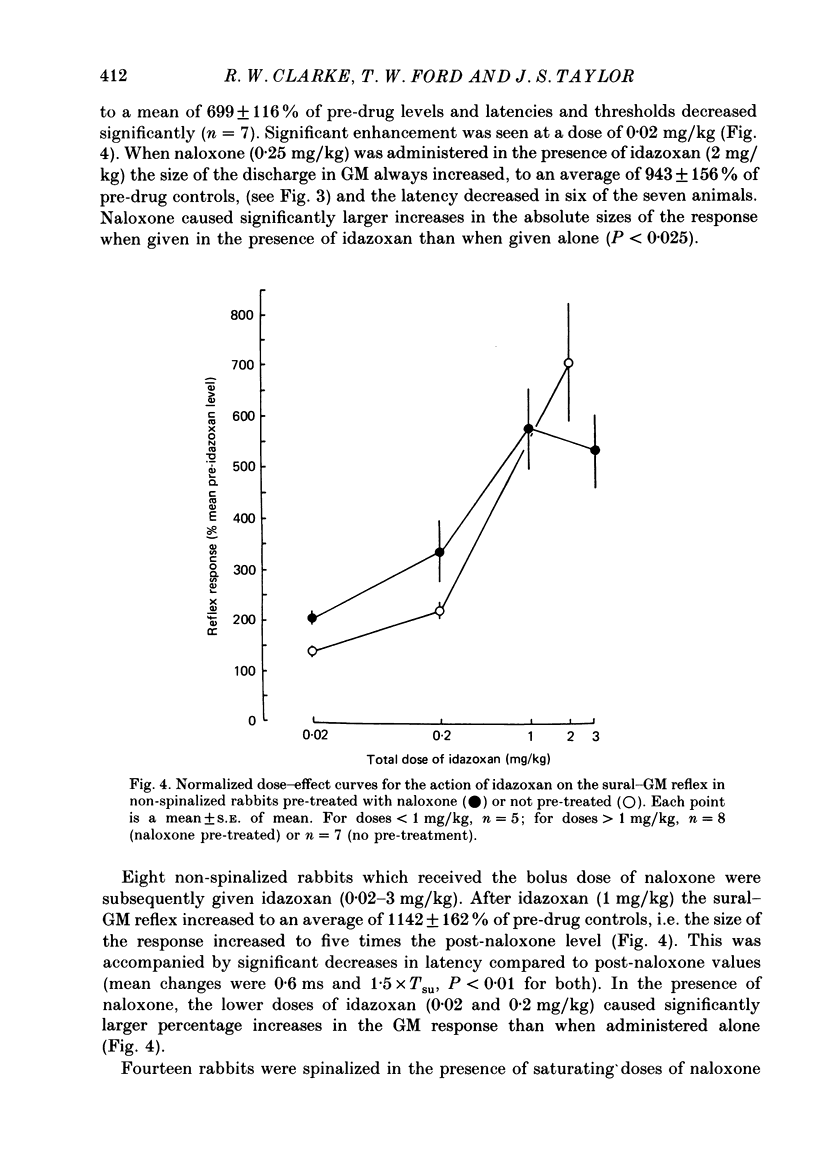
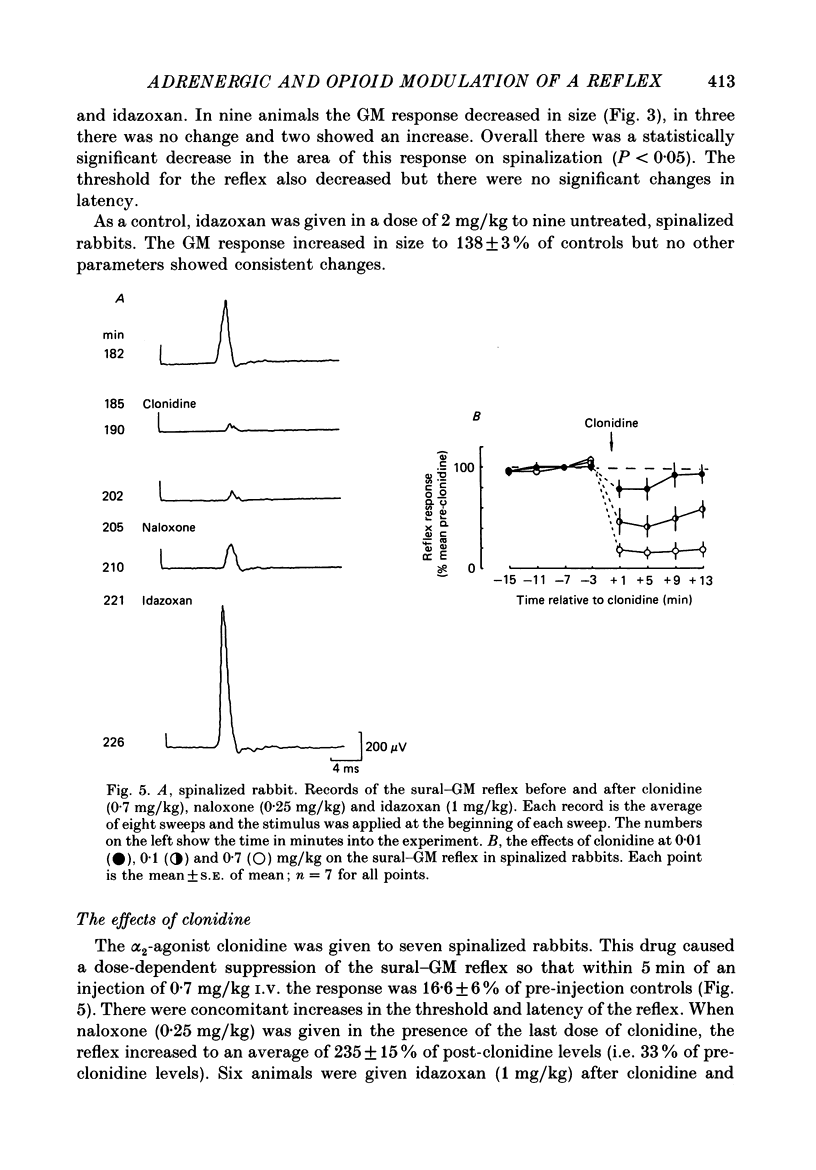
1. In the decerebrated and spinalized rabbit, electrical stimulation of the sural nerve evokes a short-latency reflex in the ipsilateral ankle extensor gastrocnemius medialis (GM) which is tonically suppressed by endogenous opioids. In the present study we have investigated the inhibitory influences affecting this reflex in non-spinalized, decerebrated rabbits. 2. In non-spinalized rabbits, the thresholds and latencies of the sural-GM reflex were significantly higher than in spinalized preparations. The opioid antagonist naloxone and the alpha-adrenoceptor antagonist idazoxan potentiated the reflex in both preparations. Naloxone was significantly more effective in spinalized rabbits whereas idazoxan had a much larger effect in non-spinalized animals. 3. When the spinal cord was sectioned in the presence of naloxone alone, the GM reflex always increased in size. An ipsilateral hemisection of the cord was as effective as total section in this respect. When the section was performed in the presence of idazoxan and naloxone, the response usually decreased in size. 4. The alpha 2-adrenoceptor agonist clonidine depressed the reflex in spinalized rabbits, an action that was reversed by idazoxan but not by naloxone. 5. These data show that in the decerebrated, non-spinalized rabbit, the sural-GM reflex is tonically suppressed by endogenous opioids, presumably acting at the segmental level, and by an ipsilateral descending pathway which involves an alpha-adrenoceptor-mediated synapse. Activity in this descending pathway masks the facilitatory effects of opioid antagonists on spinal reflexes in this preparation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. A., Martin W. R. The effect of the narcotic antagonists naloxone, naltrexone and nalorphine on spinal cord C-fiber reflexes evoked by electrical stimulation or radiant heat. Eur J Pharmacol. 1977 Mar 21;42(2):147–154. doi: 10.1016/0014-2999(77)90354-5. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Sharpe L. G., Pickworth W. B. Electrophysiologically recorded C-fiber reflexes in intact and acute decerebrate-spinal cats: absence of naloxone facilitation in intact cats. Neuropharmacology. 1985 Jun;24(6):555–559. doi: 10.1016/0028-3908(85)90063-2. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Chalmers J. P., Howe P. R. Distribution of catecholamine-containing cell bodies in the rabbit central nervous system. J Comp Neurol. 1978 May 15;179(2):407–423. doi: 10.1002/cne.901790210. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Goodchild A. K., Dampney R. A., Chalmers J. P. Cell groups in the lower brain stem of the rabbit projecting to the spinal cord, with special reference to catecholamine-containing neurons. Brain Res. 1981 Sep 21;221(1):35–55. doi: 10.1016/0006-8993(81)91062-3. [DOI] [PubMed] [Google Scholar]
- Catley D. M., Clarke R. W., Pascoe J. E. Naloxone enhancement of spinal reflexes in the rabbit. J Physiol. 1983 Jun;339:61–73. doi: 10.1113/jphysiol.1983.sp014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. W., Ford T. W. The contributions of mu-, delta- and kappa-opioid receptors to the actions of endogenous opioids on spinal reflexes in the rabbit. Br J Pharmacol. 1987 Jul;91(3):579–589. doi: 10.1111/j.1476-5381.1987.tb11251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W. Pharmacology of descending control systems. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):375–391. doi: 10.1098/rstb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Reticulospinal inhibition of transmission in reflex pathways. J Physiol. 1968 Jan;194(1):201–223. doi: 10.1113/jphysiol.1968.sp008402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden A. I., Jacobs T. P., Smith M. T., Zivin J. A. Naloxone in experimental spinal cord ischemia: dose-response studies. Eur J Pharmacol. 1984 Aug 3;103(1-2):115–120. doi: 10.1016/0014-2999(84)90196-1. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker S. M., Mitchell R., Hope P. J., Molony V., Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 1985 May 20;334(2):243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Hannah J. A., Hamilton C. A., Reid J. L. RX781094, a new potent alpha 2 adrenoceptor antagonist. In vivo and in vitro studies in the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1983 Apr;322(3):221–227. doi: 10.1007/BF00500769. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. SUPRASPINAL CONTROL OF TRANSMISSION IN REFLEX PATHS TO MOTONEURONES AND PRIMARY AFFERENTS. Prog Brain Res. 1964;12:197–221. doi: 10.1016/s0079-6123(08)60624-x. [DOI] [PubMed] [Google Scholar]
- Megens A. A., Leysen J. E., Awouters F. H., Niemegeers C. J. Further validation of in vivo and in vitro pharmacological procedures for assessing the alpha 2/alpha 1-selectivity of test compounds: (2). Alpha-adrenoceptor agonists. Eur J Pharmacol. 1986 Sep 23;129(1-2):57–64. doi: 10.1016/0014-2999(86)90336-5. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]


