Abstract
Invasive fungal infection (IFI) due to moulds other than Aspergillus are a significant cause of morbidity and mortality. Non-Aspergillus mould (NAM) infections appear to be on the increase due to an ever-expanding population of immunocompromised hosts. In this review, Mucorales, Scedosporium species, Lomentospora prolificans and Fusarium species are examined in detail, and the microbiology, risk factors, diagnosis and treatment of emerging NAMs such as Paecilomyces variotti, Purpureocillium lilacinum and Rasamsonia are summarized. The challenges in diagnosis are emphasized and the emerging importance of molecular methods is discussed. Treatment of IFI due to NAMs is a multi-pronged and multi-disciplinary approach. Surgery, correction of underlying risk factors, and augmentation of the host immune response are as important as antifungal therapy. Many of these NAMs are intrinsically resistant to the currently licensed antifungal agents, so selection of therapy needs to be guided by susceptibility testing. There are new antifungal agents in development, and these have the potential to improve the efficacy and safety of antifungal treatment in the future. Ongoing research is required to fully delineate the epidemiology of NAM infections, and to develop better diagnostic tools and treatments so that outcomes from these infections can continue to improve.
Introduction
Although Aspergillus is the major cause of invasive mould infections (70%–80%), non-Aspergillus moulds (NAMs) are more challenging to diagnose and treat and are associated with higher mortality rates.1 NAM infections appear to be increasing due to an expansion of the at-risk population, new immunotherapeutic agents available for cancer/haematological malignancy treatment, and selective pressures related to increasing use of broad-spectrum mould-active antifungal prophylaxis.2–6
In this review, the epidemiology and risk factors, diagnosis and treatment of NAMs, including Mucorales, Scedosporium spp., Lomentospora prolificans and Fusarium spp., will be examined. This review will provide clinicians with practical frameworks for the prompt diagnosis and treatment of NAMs in clinical practice and pay particular attention to cutting-edge diagnostic tools, novel antifungal agents and adjuvant therapies that are increasingly available for use in clinical practice.
Methods
To provide clinicians with comprehensive guidance on how to diagnose and manage NAM infections a literature review of PubMed was undertaken up until 30 April 2024. The following terms were used: ‘adjunct’; ‘amphotericin B’; ‘antifungal’; ‘azole’; ‘basidiomycetes’; ‘blood culture’; ‘breakthrough’; ‘central nervous system’; ‘check point inhibitors’; ‘computed’; ‘corticosteroid’; ‘COVID-19’; ‘culture’; ‘cystic fibrosis’; ‘cytotoxic T-cells’; ‘deferasirox’; ‘diagnosis’; ‘disseminated’; ‘dissemination’; ‘duration’; ‘echinocandin’; ‘emerging’; ‘endophthalmitis’; ‘epidemiology’; ‘fosmanogepix’; ‘fungemia’; ‘fusariosis’; ‘Fusarium’; ‘G-CSF’; ‘GM-CSF’; ‘graft-versus-host-disease’; ‘granulocyte transfusion’; ‘hematological malignancy’; ‘hematopoietic stem cell transplant’; ‘histology’; ‘histopathological’; ‘histopathology’; ‘hyalohyphomycosis’; ‘hyperbaric oxygen’; ‘ibrexafungerp’; ‘identification’; ‘imaging’; ‘immunocompromise’; ‘immunosuppression’; ‘invasive fungal disease’; ‘invasive fungal infection’; ‘leukemia’; ‘Lomentospora prolificans’; ‘lymphoma’; ‘MALDITOF’; ‘microscopy’; ‘MIC’; ‘mold’; ‘molecular’; ‘mortality’: ‘MRI’; ‘Mucorales’; ‘mucormycosis’; ‘myeloma’; ‘nasal’; ‘neutropenia’; ‘non-Aspergillus mold’; ‘nose’; ‘olorofim’; ‘Paecilomyces’; ‘PCR’; ‘Penicillium’; ‘phaeohyphomycosis’; ‘probable’; ‘proven’; ‘Pseudallescheria’; ‘pulmonary’; ‘Purpureocillium’; ‘Rasamsonia’; ‘response’; ‘rhino-orbital-cerebral’; ‘risk factor’; ‘salvage’; ‘SARS-CoV-2’; ‘scedosporiosis’; ‘Scedosporium’; ‘Schizophyllum commune’; ‘Scopulariopsis’; ‘sequencing’; ‘serology’; ‘sinus’; ‘skin’; ‘solid organs transplant’; ‘species’; ‘surgery’; ‘susceptibility testing’; ‘suspected’; ‘Talaromyces’; ‘terbinafine’; ‘therapeutic drug monitoring’, ‘therapy’; ‘treatment’; ‘tyrosine kinase inhibitor’; ‘VT-1161’; ‘VT 1598’; ‘zygomycetes’; ‘zygomycosis’; ‘Zygomycota’.
Mucorales
With the advent of molecular tools, a change in nomenclature has occurred, such that the term Zygomycota is now obsolete. All infections due to the order Mucorales are now termed mucormycosis and not zygomycosis.7 The most common members are Rhizopus spp., Mucor spp. and Lichtheimia spp. (formerly Absidia and Mycocladus). Other important members include Rhizomucor, Cunninghamella, Apophysomyces and Saksenaea spp. There are geographical variations in the relative frequency, with Apophysomyces most common in India but very rare in Europe.8–13
Risk factors
Risk factors that should prompt a clinician to suspect mucormycosis include advanced haematological malignancy, poor performance status, allogeneic HSCT, solid organ transplant (SOT), poorly controlled diabetes mellitus (DM) and/or diabetic ketoacidosis (DKA), iron overload and deferoxamine therapy, prolonged corticosteroid use, burns and major trauma, and prior prophylaxis with azoles and echinocandins.2,14–17 More recently, Bruton tyrosine kinase inhibitors and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been implicated as risk factors.15,18–20 Despite this, many patients with invasive mucormycosis have no identifiable risk factor. Again, geographical variations occur, with DM being the most common risk factor in India, and a haematological malignancy the most common in Europe and the USA.17
Clinical manifestations
The most common clinical manifestation of mucormycosis is rhino-orbital-cerebral (ROC) infection (proptosis, ophthalmoplegia, nasal drip/epistaxis, hemiplegia,) especially in those with poorly controlled DM and/or DKA.16 The lungs are the second most common site of mucormycosis. The most common risk factor for pulmonary mucormycosis (PM) is a haematological malignancy or HSCT, and patients commonly present with fever, pleuritic chest pain, persistent cough and haemoptysis.9,10,21 Other sites of infection include the skin, especially after major trauma, but they can also be seen in disseminated disease.22 Lesions are commonly painful, erythematous, papular/nodular with some having a central necrotic area. Gastrointestinal disease can occur in malnourished patients and in low-birthweight infants as well as in the immunocompromised (e.g. haematology patients, SOT). Symptoms include abdominal pain, diarrhoea, gastrointestinal bleeding, abdominal distension and perforation.23
Diagnosis
Diagnosis is difficult and challenging as conventional microbiological tests lack sensitivity and no specific biomarkers have been validated. As disseminated disease can occur in 16% to 40%, blood cultures should be taken, and a detailed examination of the skin should be performed to detect any suspicious lesions for biopsy.24–27 A chest CT scan should be performed in all cases of suspected PM. Signs that increase the likelihood of PM over invasive pulmonary aspergillosis include the reverse-halo sign (Figure 1), vessel-occlusion sign (contrast flow interruption), >10 nodules and a pleural effusion (Table 1).28–30 A sinus CT is recommended in all cases of suspected ROC mucormycosis and when PM is diagnosed to determine the extent of infection. Sinus CT scan findings consistent with ROC mucormycosis include mucosal thickening, sinus opacification, bony erosions (which may be late) and extension into the neighbouring tissues.67 With any extension an MRI scan should also be performed to better delineate the full extent of the infection in the brain and eyes. Imaging is also very important to identify appropriate sites for biopsy. Table 1 details the recommended tests to be used to diagnose mucormycosis.
Figure 1.

CT scan image (axial view) of a reverse halo sign. This is defined as a central area of ground-glass opacification surrounded by a denser peripheral area of consolidation. Courtesy of Professor Samantha Ellis, Department of Radiology, Alfred Health, Melbourne.
Table 1.
Diagnosis of invasive fungal infection due to Mucorales, Scedosporium species, Lomentospora prolificans and Fusarium species
| Organism | Diagnostic test | Specifics of diagnostic test | Comments | Relevant references |
|---|---|---|---|---|
| Mucorales | Blood cultures | Should be performed in every patient with suspected mucormycosis | 24–27 | |
| Imaging | CT scans of sinuses, lungs and abdomen MRI scans of brain, eyes, spine, bones and joints |
Imaging should be performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment Features characteristic of PM include vessel-occlusion sign (contrast flow interruption), >10 nodules and a pleural effusion (Figure 1) |
28–30 | |
| Microscopya | To identify genus and species | Non-pigmented; wide, ribbon-like with irregular branching; 6–16 μm in width; non-septate or pauci-septate hyphae (Figure 2) | 31 | |
| Culturea | To identify genus and species and for antifungal susceptibility testing | Avoid homogenization of tissue and incubate at 30°C and 37°C to increase yield. Depends on species but colonies are usually cottony and white to grey/brown in colour | 31 | |
| MALDI-TOF MS | Used as an adjunct for species identification | Use of validated in-house databases associated with better accuracy. Only 86% of isolates correctly identified using commercially available database in one multi-centre study | 32 | |
| Serology | GM and 1,3-β-D-glucan assays | Not recommended to use as an adjunct for the diagnosis of mucormycosis. GM is not present in the cell wall and the amount of 1,3-β-D-glucan present is below the lower limit of detection of the assay | ||
| Molecular | Adjunct for the diagnosis of mucormycosis | In-house using a variety of DNA targets (ITS, 18S rDNA, 28S rDNA) and techniques (e.g. PCR ± sequencing) Fresh tissue is preferred as sensitivity is greater than for FFPE tissue Can be used on blood, CSF and BAL fluid |
33 | |
| Mucorales-specific assays for use in serum | Sensitivity of 81% for proven or probable mucormycosis in a retrospective study of 44 patients Adjunct for the diagnosis of mucormycosis, if available |
34,35 | ||
| Susceptibility testing | CLSI EUCAST |
Should be performed on all isolates. No interpretative breakpoints. MIC values used to guide antifungal therapy | 36–38 | |
| Scedosporium spp. | Blood cultures | Should be performed in every patient with suspected scedosporiosis | ||
| Imaging | CT scans of sinuses, lungs and abdomen MRI scans of brain, eyes, spine, bones and joints |
Imaging should be performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment No specific features to differentiate it from other NAMs |
||
| Microscopya | To identify genus and species | Septate hyphae that branch at 60–70°. Conidiogenous cells are usually flask-shaped. Conidia are usually unicellular and oval-shaped. Presence of ascocarp and/or pyriform adventitious conidia helps in differentiation from other NAMs (Figure 3) | ||
| Culturea | To identify genus and species and for antifungal susceptibility testing | Grow rapidly. Darker on the reverse side (dark grey to black) as compared with the upper surface (grey to white). Mycelial tufts are fine and short. Cultures tend to become cottony with maturity (Figure 4) | ||
| MALDI-TOF MS | Used as an adjunct for species identification | Usually only successful if commercial databases supplemented with in-house libraries | ||
| Serology | GM | Not recommended to use as an adjunct for the diagnosis of scedosporiosis | 39 | |
| 1,3-β-D-glucan assays | Can be used as an adjunct for the diagnosis of fusariosis | |||
| Molecular | Adjunct for diagnosis and identification to species level | No standardized assays available. Any assays have only been well studied on CF patients. Recommend pan-fungal PCR for use on tissue specimens and ITS1, ITS2 and β-tubulin and calmodulin sequencing for use on cultures | 40–42 | |
| Susceptibility testing | CLSI EUCAST |
Should be performed on all isolates. No interpretative breakpoints. Similar results obtained with Sensititre YeastOne Y010 assay as with CLSI and EUCAST. MIC values used to guide antifungal therapy. Generally high MIC values of amphotericin B, isavuconazole and itraconazole. Lowest MIC values seen with voriconazole followed by posaconazole. Echinocandins can exhibit activity against some isolates | 37,38,43,44 | |
| Lomentospora prolificans | Blood cultures | Should be performed in every patient with suspected invasive infection due to L. prolificans (positive in up to 72%) | 45 | |
| Imaging | CT scans of sinuses, lungs and abdomen MRI scans of brain, eyes, spine, bones and joints |
Imaging should be performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment No specific features to differentiate it from other NAMs |
||
| Microscopya | To identify genus and species | Pigmented, flask-shaped conidiophores that are swollen at the bases (Figure 5) | ||
| Culturea | To identify genus and species and for antifungal susceptibility testing | Characterized by rapid growth. Colonies are olive to black in colour and the surface is suede to downy | ||
| MALDI-TOF MS | Used as an adjunct for species identification | Usually only successful if commercial databases supplemented with in-house libraries | ||
| Serology | GM | Not recommended to use as an adjunct for the diagnosis of invasive infection due to L. prolificans | ||
| 1,3-β-D-glucan assays | Can be used as an adjunct for the diagnosis of fusariosis | |||
| Molecular | Adjunct for diagnosis and identification to species level | Several non-standardized assays available (oligoarray, multiplex PCR, pan-fungal PCR and sequencing, and PCR and reverse line blot hybridization A multiplex fungal or pan-fungal PCR assay followed by sequencing, hybridization or microarray is recommended |
41,46–53 | |
| Susceptibility testing. | CLSI EUCAST |
Mostly resistant to all currently licensed antifungal agents. Some isolates have low MIC values against voriconazole and posaconazole (but less so) | 37,38,43,44 | |
| Fusarium spp. | Blood cultures | Should be performed in every patient with suspected fusariosis | 54 | |
| Imaging | CT scans of sinuses, lungs and abdomen MRI scans of brain, eyes, spine, bones and joints |
Imaging should be performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment Less likely to demonstrate halo-sign(s) than invasive Aspergillus infection |
55,56 | |
| Microscopya | To identify genus and species | Hyaline hyphae with pigmented banana- or canoe-shaped multicellular macroconidia with a foot cell at the base (Figure 7). Adventitious sporulation can help in differentiation from Aspergillus | ||
| Culturea | To identify genus and species and for susceptibility testing | Usually, colonies are pink or violet in the centre with a lighter periphery. F. solani SC differ as conidia are usually blue-green | ||
| MALDI-TOF MS | Used as an adjunct for species identification | Only available in research centres for Fusarium spp. | 57 | |
| Serology | GM and 1,3-β-D-glucan assays | Are recommended for use as an adjunct for the diagnosis of fusariosis. GM assay cross-reacts with Fusarium spp. In one study GM assay had a sensitivity of 83%, a specificity of 67% and in 73% was positive before clinical signs and symptoms developed. Prognostic value as well as persistently positive results correlate with a poor outcome When the threshold for the 1,3-β-D-glucan assay is >80 pg/mL and two sequential positive results are used to diagnose invasive fusariosis, the sensitivity is high (90%) but the specificity is low at 60% |
58
59 |
|
| Molecular | Adjunct for diagnosis and identification to species level | Several methods available: AFLP, loop-mediated isothermal amplification, MLST and RT-PCR Sequencing of the TEF1-α gene can differentiate between the different species: F. oxysporum, F. solani, F. keratoplasticum, F. petroliphilum, F. napiforme, F. falciforme, F. pseudensiforme and F. dimerum The use of TEF1-α gene sequencing with multiplex PCR or DNA microarray hybridization can detect F. solani and F. oxysporum in neutropenic patients The use of TEF1-α gene sequencing with pan-fungal PCR (ITS target) and Luminex multi-analyte profiling technology can detect F. solani, F. oxysporum, F. verticillioides, and F. proliferatum (considered a pan-Fusarium PCR assay) Can be used as in blood, CSF and tissue Needs further validation before it is used routinely in clinical practice Recommend TEF1-α gene sequencing as an adjunct for diagnosis and speciation, if available RPB2 can be recommended for use in species identification |
41,48,50,51,60–64 | |
| Susceptibility testing | CLSI EUCAST |
Usually have high MIC values to most of the currently licensed antifungal agents. However, the MIC values vary from species to species hence the importance of species identification Amphotericin B and voriconazole usually have activity against most species, except for F. solani SC and F. verticillioides (resistant to voriconazole and high MIC values of amphotericin B) Fusarium spp. are intrinsically resistant to echinocandins Correlation of clinical outcome to MIC values is unclear as data are conflicting |
37,38,65,66 |
AFLP, amplified fragment length polymorphism; BAL, bronchoalveolar lavage fluid; CF, cystic fibrosis; FFPE, formalin-fixed paraffin-embedded; GM, galactomannan; ITS, internal transcriber sequence; NAM, non-Aspergillus moulds; PM, pulmonary mucormycosis; rDNA, ribosomal DNA; RPB2, RNA polymerase II second largest subunit; RT-PCR, real-time PCR; SC, species complex.
aA detailed description of the microscopy and culture features of each NAM can be found at Mycology Online (http://www.mycology.adelaide.edu.au).
It is critically important to obtain specimens for microscopy, culture, and histopathological and/or molecular testing when mucormycosis is suspected. The type of specimen is dependent on the site of infection. For example, in suspected PM a bronchoalveolar lavage may provide the prerequisite specimens. As Mucorales are wide and ribbon-like in structure with irregular branching and non-septate or pauci-septate hyphae (Figure 2), they only grow 15%–25% of the time.31 As a result, more invasive biopsies (CT-guided, open lung) may be needed (Table 1). In this setting, it is important that clinicians liaise with the microbiology laboratory to ensure that these specimens are not minced/homogenized. This will improve the chances of a positive culture. A culture is very important for identification to species level and antifungal susceptibility testing (Table 1). Commercially available monoclonal antibodies against Mucorales may be used on histological specimens to differentiate between Mucorales and Aspergillus.68 Definitive identification is made by sequencing [internal transcriber spacer (ITS)] and MALDI-TOF MS. If histology shows features consistent with Mucorales but culture is negative, then PCR testing should be performed on the tissue specimen. Several PCR assays have been developed for the detection of Mucorales in serum and blood, with sensitivities and specificities of 75%–100% and 84.6%–100%, respectively. Some of these assays are in-house, but commercial assays are also available (Mucorgenius®, PathoNostics). Comprehensive standardization and validation are still required. A full description of these PCR assays for Mucorales is beyond the scope of this review, but Lamoth and Kontoyiannis33 provide an excellent overview of the topic. If a PCR assay is readily available, then clinicians should use them as an adjunct for diagnosis. As galactomannan (GM) is not a component of Mucorales and the amounts of 1,3-β-D-glucan produced by members of this order are below the lower limit of detection, these two serological assays are not recommended for the diagnosis of mucormycosis (Table 1).
Figure 2.

Rhizopus microsporus showing dispersed hyphae/stolon structure. Low objective view. Prepared using adhesive tape method and stained with lactophenol blue. Courtesy of Professor Wieland Meyer, Westerdijk Fungal Biodiversity Institute and Ms Krystyna Maszewska, Dr Catriona Halliday, Mr Alex Khan, Mr Tsung-Yu Pai and Ms Georgia Clementine Wunderlich, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Westmead, NSW, Australia.
Treatment
There are four key elements to the treatment of mucormycosis. These include prompt directed antifungal therapy (optimized using therapeutic drug monitoring),69–71 surgical debridement, adjuvant therapies and reversal of the underlying risk factors (e.g. treat DKA and obtain good control of DM). A delay in antifungal therapy beyond 3 days increases mortality from 33% to 72%.21 Liposomal amphotericin B (L-AMB) is still considered as the drug of choice for treatment of mucormycosis at a dose of 5 mg/kg IV daily unless there is CNS involvement, whereupon a dose of 10 mg/kg IV is recommended (Table 2).72,73 Posaconazole as modified-release tablet or IV formulations, and isavuconazole orally or IV are alternatives as first-line (if significant baseline renal impairment) or salvage therapy (if failed primary therapy with L-AMB).75,106,107 Table 2 provides details on the dosing of the different antifungal agents and the evidence base for the recommendations for treatment in different clinical scenarios. Combination therapy is not routinely recommended as first-line treatment; however, L-AMB and an echinocandin (likely a class effect) may be used in selected critically ill or severe cases (Table 2).78–80 A switch from IV to oral therapy is usually recommended once there has been a stable or partial response to treatment. The liquid suspension formulation of posaconazole is not recommended for treatment unless none of the other recommended commercially available antifungal agents are available (Table 2).108 Of all the novel antifungal agents that are in development (Table 3), fosmanogepix (targets glycosylphosphatidylinositol-anchored protein maturation by inhibiting the fungal enzyme Gwt1) appears to be the only one with in vitro activity against some organisms of the Mucorales order [minimum effective concentration (MEC) values of ≤1 mg/L against some isolates, but most have MEC values of 4–16 mg/L (Table 3)].109,110,122,123 A Phase II trial has just been terminated to make way for a Phase III trial for the same indication (i.e. examining the efficacy of fosmanogepix in the treatment of Aspergillus or rare moulds) (Table 3).
Table 2.
Treatment of invasive fungal infection due to Mucorales, Scedosporium species, Lomentospora prolificans and Fusarium species
| Organism | Antifungal regimen | Formulations, dosing, route | Comments | Relevant references | |
|---|---|---|---|---|---|
| Mucorales | First-line: Recommended Alternative(s) Salvage: Recommended Alternative(s). |
L-AMB Posaconazolea Isavuconazolea,b ABLC L-AMB plus caspofungin Posaconazolea Isavuconazolea,b L-AMB ABLC L-AMB plus caspofungin L-AMB plus posaconazolea Posaconazole suspensiona |
5 mg/kg IV daily MR tablets/IV; 300 mg twice daily for two doses then 300 mg once daily thereafter 200 mg PO/IV three times a day for 2 days then 200 mg PO/IV daily thereafter 5 mg/kg IV daily L-AMB: 5 mg/kg IV daily Caspofungin: 70 mg IV daily on Day 1 then 50 mg IV daily thereafter MR tablets/IV; 300 mg twice daily for 2 doses then 300 mg once daily thereafter 200 mg PO/IV three times a day for 2 days then 200 mg PO/IV daily 5 mg/kg IV daily 5 mg/kg IV daily L-AMB: 5 mg/kg IV daily Caspofungin 70 mg IV daily on Day 1 then 50 mg IV daily thereafter L-AMB: 5 mg/kg IV daily Posaconazole: MR tablets/IV; 300 mg twice daily for two doses then 300 mg once daily thereafter Posaconazole suspension: 400 mg PO twice daily 400 mg PO twice daily |
10 mg/kg IV daily if CNS disease If baseline renal impairment MoveOn Study compared first-line posaconazole (MR tablets/IV) with L-AMB + posaconazole (MR tablets/IV) with L-AMB alone Day 42 favourable response: 80% (4/5) vs 27.8% (5/18) vs 20% (3/15), respectively If baseline renal impairment VITAL trial: proven or probable mucormycosis cases treated with first-line isavuconazole c/w with external controls from the FungiScope Registry EOT favourable responses 32% (6/19) in cases Day 42 crude all-cause mortality 33% vs 39% (weighted all-cause mortality 33% vs 41%; P = 0.595) Not recommended in CNS disease L-AMB 10 mg/kg IV daily if CNS disease Consider continuing with 70 mg IV daily of caspofungin beyond Day 1 in critically ill patients and in those who weigh >80 kg Can use any echinocandin as likely a class effect Significantly greater treatment success of ROCM with the combination c/w polyene monotherapy (100% vs 45%; P = 0.02) Combination recommended only if severe disease or in a critically ill patient Where primary treatment with L-AMB has failed Where primary treatment with L-AMB has failed 10 mg/kg IV daily if CNS disease If primary therapy with posaconazole or isavuconazole has failed If primary therapy with posaconazole or isavuconazole has failed Not to be used in cases with CNS disease L-AMB 10 mg/kg IV daily if CNS disease Consider continuing with 70 mg IV daily of caspofungin beyond Day 1 in critically ill patients and in those who weigh >80 kg Can use any echinocandin as likely a class effect Combination recommended only if severe disease or in a critically ill patient L-AMB 10 mg/kg IV daily if CNS disease Any formulation of posaconazole can be used but prefer MR tablets/IV if available Cases of proven or probable mucormycosis identified from two registries (SEIFEM and FungiScope): 56% (18) treated with combination as salvage therapy had a favourable response Only if limited availability of other antifungal agents |
72–74
75 76 77 78–80 75 76 77 78–80 81 |
| Scedosporium spp. | First-line:Recommended Alternative(s) Salvage:Recommended Alternative(s) |
Voriconazolea Posaconazolea Posaconazolea plus terbinafine Voriconazolea Addition of an echinocandin and GM-CSF to pre-existing treatment with voriconazolea Posaconazolea |
6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter MR tablets/IV; 300 mg twice daily for two doses then 300 mg once daily thereafter Posaconazole: MR tablets/IV; 300 mg twice daily for two doses then 300 mg once daily thereafter Terbinafine: 250 mg PO twice daily 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Caspofungin 70 mg IV daily on Day 1 then 50 mg IV daily thereafter Or Anidulafungin 200 mg IV daily on Day 1 then 100 mg IV daily thereafter Or Micafungin 100 mg IV daily MR tablets/IV; 300 mg twice daily for two doses then 300 mg once thereafter |
IV treatment is recommended initially. Step-down to oral therapy once clinically stable When MIC values of posaconazole are high, extrapolating from the treatment of L. prolificans Failed or intolerant of L-AMB or posaconazole given as primary treatment Consider continuing with 70 mg IV daily of caspofungin beyond Day 1 in critically ill patients and in those who weigh >80 kg Consider increasing the dose by 50%–75% beyond Day 1 in critically ill patients Consider increasing all doses by 50% in obese patients Consider using 150 mg daily in critically ill patients Seek expert advice from a haematologist regarding dosage of GM-CSF |
82,83
46,83–87 88 89 90 |
| Lomentospora prolificans | First-line:Recommended Alternative(s) Salvage:Recommended |
Voriconazolea and terbinafine Voriconazolea plus L-AMB Voriconazolea plus micafungin Voriconazolea Voriconazolea-based combination regimen |
Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Terbinafine: 250 mg PO twice daily Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter L-AMB: 5 mg/kg IV daily Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Micafungin: 100 mg IV daily 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter |
Step-down to oral therapy once clinically stable 45% (8/18) were alive at Day 42 and the probability of survival was significantly greater in those receiving the combination Only a small number of cases treated with this combination 10 mg/kg IV daily if CNS disease Only a small number of cases treated with this combination Consider using 150 mg daily in critically ill patients As monotherapy if terbinafine is not available The remainder of the regimen is dependent on the response to, or intolerance of other prior antifungal agents used. Seek expert advice |
83–87,91,92
83,87,91 83,87,91 82,91,93,94 |
| Fusarium spp. | First-line: Recommended Alternative(s) Salvage: Recommended Alternative(s) |
Voriconazolea/L-AMB/ABLC Voriconazolea plus L-AMB/ABLC Voriconazolea Voriconazolea-based combination regimen Posaconazolea L-AMB or ABLC |
Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter L-AMB/ABLC: 5 mg/kg IV daily Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter L-AMB/ABLC: 5 mg/kg IV daily Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Voriconazole: 6 mg/kg PO/IV twice daily for two doses then 4 mg/kg PO/IV twice daily thereafter Posaconazole: MR tablets/IV; 300 mg twice daily for two doses then 300 mg once thereafter 5 mg/kg IV daily |
Voriconazole or lipid formulations of amphotericin B cannot be recommended one over the other IV voriconazole is recommended initially with step-down to oral once clinically stable Global study of 236 patients: 90 day probability of survival was 53% in those treated with voriconazole and 48% in those treated with a lipid formulation of amphotericin B L-AMB: 10 mg/kg IV daily if CNS disease ABLC: not recommended in CNS disease One lipid formulation cannot be recommended over the other Use combination in critically ill patients pending on MIC values. De-escalate to monotherapy once MIC values are known IV voriconazole is recommended as initial therapy L-AMB: 10 mg/kg IV daily if CNS disease ABLC: not recommended in CNS disease If refractory to a lipid formulation of amphotericin B IV voriconazole is recommended as initial therapy with step-down to oral once clinically stable Options: Lipid formulation of amphotericin B ± terbinafine L-AMB/ABLC: 5 mg/kg IV daily L-AMB: 10 mg/kg IV daily if CNS disease ABLC: not recommended in CNS disease Terbinafine: 250 mg twice daily Fusarium spp. usually have high MIC values against posaconazole Need to know susceptibility results before use. As a result, it is only recommended as salvage therapy and only as the MR tablet or IV formulation L-AMB: 10 mg/kg IV daily if CNS disease ABLC: Not recommended in CNS disease Used if refractory to or intolerant of voriconazole |
56,95–97
77 96,98–101 101 101 99,102–104 77 |
ABLC, amphotericin B lipid complex; c/w, compared with; EOT, end of therapy; L-AMB, liposomal amphotericin B; MR, modified-release; PO, oral; ROCM, rhino-orbital-cerebral mucormycosis; SEIFEM, Sorveglianza Epidemiologica Infezioni Fungine nelle Empoatie Maligne.
aTherapeutic drug monitoring recommended. Aim for a level of ≥1 mg/L for voriconazole, posaconazole and isavuconazole.69–71,105
b200 mg of isavuconazole is equal to 372 mg of isavuconazonium sulphate (prodrug of isavuconazole).
Table 3.
Novel antifungal agents in development with potential to treat invasive fungal infection due to Mucorales, Scedosporium species, Lomentospora prolificans and Fusarium species
| Antifungal agent | Mode of action |
In vitro activity (n)a |
In vivo activitya | Clinical trials | Relevant references |
|---|---|---|---|---|---|
| Fosmanogepix (formerly APX001) | GPI-anchor inhibitor |
Mucorales
Cunninghamella bertholletiae (10) MEC90 > 8b Lichtheimia spp. (20) MEC90 > 8b Mucor circinelloides (10) MEC90 ≤ 2–8b Rhizopus spp. (30) MEC90 > 8b Scedosporium spp. S. apiospermum (38) MEC90 0.12–16b S. aurantiacum (10) MEC90 0.03b S. boydii (10) MEC90 0.12b Lomentospora prolificans (38) MEC90 0.06–0.12b Fusarium spp. F. oxysporum (25) MEC90 0.25–16b F. solani (15) MEC90 0.06b F. verticilloides (10) MEC90 16b |
Mucorales
Mouse model using strains with MEC values of 0.25 and 4.0 Fungal burden decreased by 1.3 and 1.97 log10 Ce/g lung tissue when dosed with 78 mg/kg (plus ABT) and 104 mg/kg (plus ABT) of FMGX, respectively. Potentially treatable with FMGX if MEC values are low Scedosporium spp. Significant increase in median survival from 7 (placebo) to 13 and 11 days in mice treated with 78 mg/kg (plus ABT) daily and 104 mg/kg (plus ABT) daily of FMGX, respectively Fusarium spp. Significant increase in median survival from 7 (placebo) to 12 and 10 days in mice treated with 78 mg/kg (plus ABT) daily and 104 mg/kg (plus ABT) daily of FMGX, respectively |
Phase II trial completed. Evaluating Aspergillus and rare moulds. No results reported (NCT04240886) |
109–113
111,112,114 111,112 111,112,114 |
| Olorofim (formerly 901318) |
Fungal dihydroorotate dehydrogenase inhibitor |
Mucorales
No activity due to phylogenetic differences in the DHODH drug target Scedosporium spp. S. apiospermum (30) MIC90 0.25b Range 0.03–0.5b S. aurantiacum (20) MIC90 1b Range 0.06–1b S. boydii (30) MIC90 0.25b Range 0.06–0.5b Lomentospora prolificans (30) MIC90 0.5.b Range 0.06–0.5.b Fusarium spp. F. dimerum (2) MIC50 >2b Range 2 to >2b F. moniliforme (1) MIC50 0.03b F. oxysporum (5) MIC50 2b Range 0.12 to >2b F. solani (11) MIC50 >2b Range 2 to >2b F. verticilloides (1). MIC90 0.5.b |
Neutropenic mouse model treated with olorofim (15 mg/kg, q8h) Survival in mice infected with S. apiospermum, P. boydii and L. prolificans at Day 10 post-infection was 80%, 100% and 100%, respectively c/w 20% in the untreated controls Decreased fungal burden in kidneys Day 3 post-infection |
Phase IIb trial: Evaluating treatment of Aspergillus, Coccidioides and rare mould infections in patients with limited or no other options Successful EORTC-MSGERC overall responses at Day 42 and Day 84 were 55% and 36% for Scedosporium (n = 11) and 53% and 53% for L. prolificans (n = 17) (NCT03583164) |
115,116
117 |
| Ibrexafungerp (formerly SCY078/MK-3118) |
Triterpenoid antifungal. Inhibits 1,3-β-D-glucan synthase |
Mucorales
No activity Scedosporium spp. S. apiospermum/P. boydii (19) MEC90 4b Range 1–8b Lomentospora prolificans (5) MEC90 4b Range 1–4b Fusarium spp. No activity |
Immunosuppressed mouse model infected with Rhizopus delemar (mucormycosis). Treated with ibrexafungerp (30 mg/kg PO twice daily) Survival to Day 21 significantly better in mice treated with ibrexafungerp c/w placebo (P < 0.002) and was equivalent to other antifungal agents. Treatment with ibrexafungerp and L-AMB resulted in significantly improved survival c/w monotherapy (P < 0.04). No in vitro activity (MEC >8) but has in vivo activity |
Phase II trial (FURI) Evaluating the treatment of patients with IFI who are intolerant of or refractory to other standard antifungal agents Single arm, non-comparator and open-label with the primary outcome assessment of global response up to Day 180 of treatment with ibrexafungerp Trial is ongoing (NCT03059992) |
118,119
118 118 118 |
| Oteseconazole (formerly VT-1161) |
Tetrazole that selectively inhibits fungal CYP51 |
Mucorales
Rhizopus arrhizus var. arrhizus (7) MIC range 0.25–2b R. arrhizus var. delemar (5) MIC range 8 to >32 |
Mucorales
Immunosuppressed mouse model of pulmonary infection with R. arrhizus var. arrhizus (MIC 1). Median survivals for mice treated with placebo, high-dose L-AMB, VT-116 7.5 mg/kg and VT-115 15 mg/kg were 5, 8, 8 and 9 days, respectively |
120 | |
| VT-1598 | Selectively inhibits fungal CYP51 |
Mucorales
R. arrhizus (11) GM MIC 3.53b Range 0.5 to >16bc |
121 |
ABT, 1-Aminobenzotriazole; Ce, conidial equivalents; c/w, compared with; CYP51, cytochrome P51; DHODH, dihydroorotate dehydrogenase; EORTC-MSGERC, European Organization for Research and Treatment of Cancer—Mycoses Study Group Education and Research Consortium; FMGX, fosmanogepix; GM, galactomannan; GPI, glycosylphosphatidylinositol; IFI, invasive fungal infection; L-AMB, liposomal amphotericin B; MEC, minimum effective concentration; MEC90, 90% minimum effective concentration; MIC50, MIC required to inhibit the growth of 50% of isolates; MIC90, MIC required to inhibit the growth of 90% of isolates; PO, oral.
aValues are mg/L.
bTested using CLSI methodology.
cFour isolates had MIC >16 mg/L and three of the four isolates were identified as R. arrhizus var. delemar by internal transcriber sequence (ITS) sequencing.
Surgical debridement, where feasible, is strongly recommended as survival rates are higher in those who have surgery with complete resection as compared with those who do not (Table 4).6,8–10,28,124–126 Early consultation with surgical colleagues and a multidisciplinary approach to the management of mucormycosis is key to optimizing survival. Repeated surgical debridement to ensure complete resection may be required in some cases. Adjunctive therapies such as hyperbaric oxygen, growth factors and granulocyte infusions can only be recommended on a case-by-case basis as the data are conflicting or limited to case series or single reports.24,27,28,128–131 It is also difficult to determine their true efficacy as they are often given in combination with antifungal therapy and surgery (Table 4). Novel adjunctive therapies include check-point inhibitors (e.g. nivolumab) with or without IFN-γ, antibodies against the peptides of CotH (protein kinases that play a role in morphogenesis, stress adaptation and virulence), and antibodies against integrin-β1, which hold promise for the future treatment of mucormycosis.143–146 R. oryzae-specific cytotoxic T cells have been shown to have in vitro activity, but use is limited to case reports (Table 4).135 More data are required before these can be recommended for widespread use in clinical practice.
Table 4.
Adjunctive treatments for invasive fungal infection due to Mucorales, Scedosporium species, Lomentospora prolificans and Fusarium species
| Organism | Adjunctive treatment | Comments | Relevant references |
|---|---|---|---|
| Mucorales | Surgical debridement | To completely resect devitalized tissue. Greatest level of evidence is for Mucorales (of all the NAMs). Multiple studies showing improved survival. Multiple surgical operations may be required | 6,8–10,28,124–126 |
| Line removal | To remove an ongoing source of infection. Recommended to remove all indwelling catheters in patients with fungaemia, if feasible. Extrapolated from data on candidaemia | 127 | |
| Growth factors: G-CSF/GM-CSF |
To augment host response. True efficacy is difficult to ascertain as given with other treatments. As a result, the data are conflicting, with one study showing no increase in survival [6/38 (21%) with G-CSF versus 2/9 (22%) in those who were not given G-CSF] and another reporting survival in 83% of cases of mucormycosis | 24,28 | |
| Granulocyte infusions | To use the infusions as a bridge until the patient’s own neutrophils recover. Given with other treatments so true efficacy is difficult to ascertain; 2/7 (29%) who received granulocyte transfusions survived in one retrospective study | 27,28 | |
| Hyperbaric oxygen | To inhibit fungal growth (in vitro models). Case reports/case series in patients with mucormycosis showing an association with improved survival: 4/7 (57.1%) who were not treated with hyperbaric oxygen died c/w 2/6 (33%) of those who were treated with hyperbaric oxygen in one retrospective study. Better outcomes for mucormycosis cases where DM is the underlying disease c/w haematological malignancy: 94% survival in those who had DM and got hyperbaric oxygen c/w 33% (P = 0.02) of those who had an underlying haematological malignancy or BMT and got hyperbaric oxygen. Biases are likely present | 128–131 | |
| DM | To treat the underlying risk. Hyperglycaemia promotes fungal proliferation and impairs phagocytosis and chemotaxis. DKA temporarily disrupts the ability of transferrin to bind iron, decreasing a key host defence, allowing Mucorales to proliferate. Reverse any DKA and achieve good control of DM | 132–134 | |
| Adaptive immunotherapy | To augment host immune response. Anti-Rhizopus oryzae T cells can be produced that increase the activity of host phagocytes. Cross-react with some but not all of the organisms of the Mucorales order | 135 | |
| Scedosporium spp. | Surgical debridement | To completely resect devitalized tissue. No data specific to invasive infection due to Scedosporium spp. in terms of improved outcomes. Extrapolated from data on Mucorales and other NAMs. Multiple surgical operations may be required (93% of cases in one study) | 136 |
| Line removal | To remove an ongoing source of infection. Recommended to remove all indwelling catheters in patients with fungaemia, if feasible. Extrapolated from data on candidaemia | ||
| Growth factors: G-CSF/GM-CSF |
To augment host response. True efficacy is difficult to ascertain as given with other treatments. GM-CSF may augment PMN oxidative burst against Scedosporium spp. | ||
| Hyperbaric oxygen | To inhibit fungal growth (in vitro models). No data specific to invasive infection due to Scedosporium spp. Extrapolated from data on Mucorales | ||
| DM | To treat the underlying risk. No data specific to invasive infection due to Scedosporium spp. Extrapolated from data on Mucorales | ||
| Lomentospora prolificans | Surgical debridement | To completely resect devitalized tissue. Associated with improved survival in cases of invasive infection due to L. prolificans (5% vs 30%; P = 0.045). Multiple surgical operations may be required | 91 |
| Line removal | To remove an ongoing source of infection. Recommended to remove all indwelling catheters in patients with fungaemia, if feasible. Extrapolated from data on candidaemia | ||
| Growth factors: G-CSF/GM-CSF |
To augment host response. G-CSF in combination with L-AMB was associated with improved survival c/w L-AMB monotherapy in a murine model (9.1 vs 13.2 days). GM-CSF in combination with posaconazole decreased the burden of infection in some organs [brain: 3.41 ± 0.10 (control) vs 3.23 ± 0.14 (POS-GM-CSF) log cfu/g; P < 0.05) but did not improve survival [7.0 ± 0.35 (controls) vs 7.3 ± 0.33 days (POS-GM-CSF); P = NS] in a murine model. G-CSF use in human survivors of invasive infection due to L. prolificans has been described. In one study of 109 patients, 20 (18.3%) were given G-CSF and 6 survived (30%). A case series from France reported that all five neutropenic patients given G-CSF survived | 45,82,137–139 | |
| Hyperbaric oxygen | To inhibit fungal growth (in vitro models). No data specific to invasive infection due to L. prolificans. Extrapolated from data on Mucorales | ||
| DM | To treat the underlying risk. No data specific to invasive infection due to L. prolificans. Extrapolated from data on Mucorales | ||
| Fusarium spp. | Surgical debridement | To completely resect devitalized tissue. Associated with improved survival in cases of invasive infection due to Fusarium spp.: 5/6 (83.3%) patients who had skin lesions resected survived Multiple surgical operations may be required. Cases of endophthalmitis need to be co-managed with an experienced ophthalmologist | 100,140 |
| Line removal | To remove an ongoing source of infection. Recommended to remove all indwelling catheters in patients with fungaemia, if feasible. Extrapolated from data on candidaemia | ||
| Growth factors: G-CSF/GM-CSF |
To augment host response. Patients administered G-CSF had a response rate of 41% | 141 | |
| Granulocyte transfusion. | To use the infusions as a bridge until the patient’s own neutrophils recover: 10/11 (91%) patients treated with granulocyte transfusions in addition to antifungals had a favourable response | 142 | |
| Hyperbaric oxygen | To inhibit fungal growth (in vitro models). Extrapolated from data on Mucorales | ||
| DM | To treat the underlying risk. No data specific to invasive infection due to Fusarium spp. Extrapolated from data on Mucorales |
BMT, bone marrow transplant; c/w, compared with; DKA, diabetic ketoacidosis; DM, diabetes mellitus; G-CSF, granulocyte colony-stimulating factor; L-AMB, liposomal amphotericin B; NAM, non-Aspergillus moulds; NS, non-significant; PMN, polymorphonuclear; POS, posaconazole.
Scedosporium species
Although ubiquitous, these saprophytic hyaline moulds are mostly found in temperate climates (e.g. Australia) resulting in geographical variations in incidence.46,147,148 The most common species include S. apiospermum species complex (SC), S. aurantiacum and S. boydii (formerly Pseudallescheria boydii).46,149
Risk factors
Until recently, Lomentospora prolificans (formerly S. prolificans) was classified with other Scedosporium spp.149 Thus, much of the epidemiological data are intertwined, making it difficult to tease out specific risk factors for Scedosporium spp. vis-à-vis L. prolificans.
Severe neutropenia and T cell immunodeficiency have been identified as risk factors, and scedosporiosis is found more commonly in patients with a haematological malignancy or post-allogeneic HSCT, especially those with graft-versus-host disease (GVHD).93
Diagnosis
As Scedosporium spp. commonly disseminate, blood cultures and a detailed examination of the skin with biopsy of any suspicious lesion should be performed (Table 1). Like other NAMs, imaging is performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment. There are no characteristic imaging lesions that distinguish scedosporiosis from other NAM infections (Table 1). Microscopy, culture, histology and molecular tests are central to the accurate and timely diagnosis of scedosporiosis (Table 1 and Figures 3 and 4). Culture is generally required to identify the isolate to species level and for susceptibility testing (Table 1). Currently available molecular assays are not standardized or species-specific and have only been studied in the cystic fibrosis (CF) population (Table 1).40 Pan-fungal PCR assays can be used on tissue specimens (sensitivity of 94.4%) and ITS1, ITS2 and β-tubulin and calmodulin sequencing of cultures are recommended as an adjunct for diagnosis, if available (Table 1).40–42 GM assays do not detect Scedosporium spp. so are not recommended for use in diagnosis (Table 1). However, a meta-analysis demonstrated that 1,3-β-D-glucan assays have a sensitivity of 80.0% for the detection of invasive scedosporiosis in serum. Sixteen cases of proven or probable invasive scedosporiosis were included in the meta-analysis.39 As such, 1,3-β-D-glucan testing can be recommended as part of the diagnostic work-up for invasive scedosporiosis.
Figure 3.
Single-forming anelloconidia of Scedosporium apiospermum attached to conidiophore, showing anellations (arrow) leading to the truncated base of the anelloconidia. Stained using lactophenol blue under coverslip. Courtesy of Professor Wieland Meyer, Westerdijk Fungal Biodiversity Institute and Ms Krystyna Maszewska, Dr Catriona Halliday, Mr Alex Khan, Mr Tsung-Yu Pai and Ms Georgia Clementine Wunderlich, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Westmead, NSW, Australia.
Figure 4.
Top of Scedosporium apiospermum colony after 4 days of growth at 27°C on Sabouraud dextrose agar. Courtesy of Professor Wieland Meyer, Westerdijk Fungal Biodiversity Institute and Ms Krystyna Maszewska, Dr Catriona Halliday, Mr Alex Khan, Mr Tsung-Yu Pai and Ms Georgia Clementine Wunderlich, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Westmead, NSW, Australia.
Treatment
Again, a multi-pronged approach to treatment is required to optimize outcomes. First-line antifungal treatment is with voriconazole (IV; 6 mg/kg 12-hourly for two doses then 4 mg/kg 12-hourly thereafter) (Table 2).82,83 Recommended second-line treatment is with posaconazole (modified-release tablets/IV) with/without terbinafine (depending on the MIC value of posaconazole) (Table 2).46,83–87 An echinocandin or GM-CSF can be added to prior voriconazole monotherapy for combination salvage therapy with reported successful outcomes (Table 2).89 Once a patient is clinically stable or a partial response is detected then step-down from IV to oral therapy is recommended. Table 2 gives a detailed description of all the recommended antifungal regimens.
Novel antifungal agents that appear to have efficacy for scedosporiosis include fosmanogepix and olorofim (Table 3). MEC values for fosmanogepix against Scedosporium spp. are as low as MEC90 0.03 mg/L (Table 3).111,112 Olorofim inhibits fungal dihydroorotate dehydrogenase, consequently inhibiting pyrimidine and DNA synthesis. Low MIC values against Scedosporium spp. have been detected by susceptibility testing (Table 3).115 A Phase IIb single-arm study of olorofim (NCT03583164) in the treatment of 202 patients with proven invasive Aspergillus or NAM infections intolerant of, resistant to or refractory to commercially available antifungal agents showed a complete or partial response of 36.4% (8/22) at Day 42%, and 22.7% (5/22) at Day 84 for infections with Scedosporium spp.150 Ibrexafungerp (a semi-synthetic derivative of enfumafungin that inhibits 1,3-β-D-glucan biosynthesis by a different pathway to echinocandins) has detected MIC values of 1–8 mg/L against Scedosporium spp. (Table 3).118
Surgical debridement, particularly of localized lesions in skin, bones and joints, is recommended (Table 4). One study reported that in 93% of cases multiple debridement was required.148 In all cases of fungaemia, it is strongly recommended that IV lines are removed (if feasible) (Table 4). This recommendation is extrapolated from the evidence on candidaemia. GM-CSFs augment human leucocyte activity against Scedosporium spp. (Table 4).136 There are no data on the use of hyperbaric oxygen to treat invasive scedosporiosis.
Lomentospora prolificans
L. prolificans is a dematiaceous hyphomycete that has recently been determined to be phylogenetically distinct from Scedosporium spp.149,151 It is found in the soil of hot and dry climates, so its incidence is higher in Australia, Spain and the USA.46,148,152
Risk factors
Risk factors include a haematological malignancy and severe neutropenia.148 Recipients of an HSCT are more likely than SOT patients to have invasive infection (39% versus 17%; P = 0.045) and for it to be fungaemic/disseminated.82 Breakthrough infections on voriconazole prophylaxis have also been identified, but are less common than for Mucorales.45,153,154
Diagnosis
As L. prolificans commonly disseminates, blood cultures and a detailed examination of the skin with biopsy of any suspicious lesion should be performed (Table 1). Like other NAMs, imaging is critical to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment (Table 1). There are no characteristic imaging lesions that distinguish lomentosporiosis from other NAM infections. Specimens for microscopy, culture, histology and molecular testing are critical to early and accurate diagnosis (Table 1). On direct microscopy L. prolificans has pigmented hyphae (Figure 5). L. prolificans can be differentiated from Scedosporium spp. as it does not grow in cycloheximide.149 Several molecular assays are available (Table 1).41,47–52 None of these are standardized and have only been examined in CF patients. Until standardization occurs, the recommended format is a broad based (e.g. pan-fungal) assay with subsequent sequencing, hybridization or microarray (Table 1).41,46–51,53 Serological testing with GM is not recommended as it cannot be detected in the cell wall of L. prolificans (Table 1). Ten cases of proven or probable invasive fungal infection (IFI) due to L. prolificans were examined using 1,3-β-D-glucan assays in serum, demonstrating a sensitivity of 81.2%.39 Thus, 1,3-β-D-glucan testing can be recommended as part of the diagnostic work-up for IFI due to L. prolificans.
Figure 5.
Histological specimen showing pigmented hyphae of Lomentospora prolificans. Grocott–Gömöri methenamine silver stain. ×100 magnification. Courtesy of Professor Catriona McLean, Anatomical Pathology, Alfred Health, Melbourne, Australia.
Treatment
A multi-pronged approach to treatment is also recommended for invasive infection due to L. prolificans. First-line antifungal treatment is with voriconazole and terbinafine (Table 2).83–87,91,92 Second-line therapy is voriconazole-based (e.g. voriconazole plus L-AMB, voriconazole plus micafungin) (Table 2).83,87,91 Table 2 gives a detailed description of all the recommended antifungal regimens. Olorofim is the first antifungal agent that has been shown to be effective against L. prolificans with low median MIC values detected (Table 3).115 The Phase IIb single-arm study of olorofim (NCT03583164) showed a complete or partial response of 42.3% at Days 42 and 84, respectively, for L. prolificans invasive infections.150 Fosmanogepix also has activity against L. prolificans, with an MEC90 of 0.06–0.12 mg/L. MIC values for ibrexafungerp are higher at 1–4 mg/L (Table 3).111,112
Surgical debridement has been associated with an improved survival (30% versus 5%; P = 0.045).91 it is particularly effective in the setting of localized lesions of the skin, bone or joints (Table 4). Lines should be removed in the setting of fungaemia (if feasible). In a murine model of disseminated infection, the addition of granulocyte (G)-CSF to amphotericin B was associated with improved survival (Table 4).137 Survival of cases where G-CSF has been used has been reported.45,82,138 The addition of GM-CSF to posaconazole reduced the burden of infection in some organs in a murine model of disseminated infection but had no impact on survival.139 As such, where available, G-CSF/GM-CSF can be recommended as adjunct therapy for the treatment of L. prolificans infection, particularly in those cases of prolonged neutropenia (Table 4). There are no data on the use of granulocyte transfusions in patients with invasive lomentosporiosis; however, extrapolating from other NAM infections they can be considered in neutropenic patients whilst awaiting recovery of their own neutrophils (Table 4).
Fusarium species
Fusarium spp. are hyaline moulds that are found mainly in soil. There are over 300 phylogenetically distinct species, which make up approximately 20 SCs.155 Ten SCs are associated with human infection, with the most common being F. solani SC (40%–60%), F. oxysporum SC (approximately 20%), F. fujikuroi SC (10%) and F. moniliforme SC (10%).156 Overall, Fusarium is the second most common NAM; however, it predominates in tropical and subtropical areas such as Brazil (13.1/1000 patients with acute myeloid leukaemia).157,158
Risk factors
Risk factors include haematological malignancy, particularly those with advanced disease and poor performance status.159 Allogeneic HSCT recipients are at risk too, especially those with T cell impairment (e.g. with GVHD).160 Dissemination is common as the fungus can undergo adventitious sporulation in tissue and blood and it is intrinsically resistant to many antifungal agents, contributing to the high mortality rates seen.95
Clinical manifestations
Paronychia is common with this fungus, so in addition to performing blood cultures and a skin examination, the nails need to be closely examined (Table 1).161 This may identify additional sites for swabbing and biopsy. Endophthalmitis can occur in the setting of dissemination, so in cases where fusariosis is suspected an ophthalmoscopic examination should be performed (Table 1). If endophthalmitis is detected then the appropriate ophthalmic specimens should be collected for microscopy, culture, histological examination and molecular testing. Consultation with an ophthalmologist is critical.
Diagnosis
CT scans of the chest are less likely to show halo-sign(s) than in invasive aspergillosis (Table 1).55,56 Other imaging should be performed to determine the sites and extent of infection, detect any suitable sites for biopsy and assess responses to treatment. Culture should be performed for full species identification.162 If Fusarium is suspected, then histology specimens should be stained with periodic acid–Schiff and Grocott–Gömöri methenamine stains as they assist in the identification of the hyphae of Fusarium spp. (Figure 6). Microscopically the hyaline hyphae with pigmented banana- or canoe-shaped multicellular macroconidia with a foot cell at the base are characteristic (Figure 7). Adventitious sporulation can be seen on microscopy, which differentiates Fusarium from Aspergillus spp. Several molecular methods are available.163–166 Sequencing of the TEF1-α gene can differentiate between the different species, and when combined with multiplex PCR or DNA microarray hybridization it can accurately identify F. solani and F. oxysporum in neutropenic patients (Table 1).48,51,60,61 The combination of TEF1-α with pan-fungal (ITS) PCR creates a pan-Fusarium assay that can be used in a variety of tissues including blood and CSF (Table 1).41,50,51,62,63 Species identification is very important as antifungal susceptibility is species dependent.65 The second largest subunit (RPB2) of RNA polymerase II has specific utility for species identification.64 Serological assays have utility in the diagnosis of fusariosis (Table 1). Aspergillus GM assay cross-reacts with Fusarium with a sensitivity and specificity of 83% and 67%, respectively.58 In most cases (73%) it detects infection before signs and symptoms are present.58 1,3-β-D-glucan assay has a sensitivity and specificity of 90% and 60%, respectively.59 If readily available (short turnaround time) then these serological tests can be used as an adjunct in the diagnosis of invasive fusariosis (Table 1).
Figure 6.

Histological specimen showing pigmented hyphae of Fusarium. Grocott–Gömöri methenamine silver stain. ×100 magnification. Courtesy of Professor Catriona McLean, Anatomical Pathology, Alfred Health, Melbourne, Australia.
Figure 7.

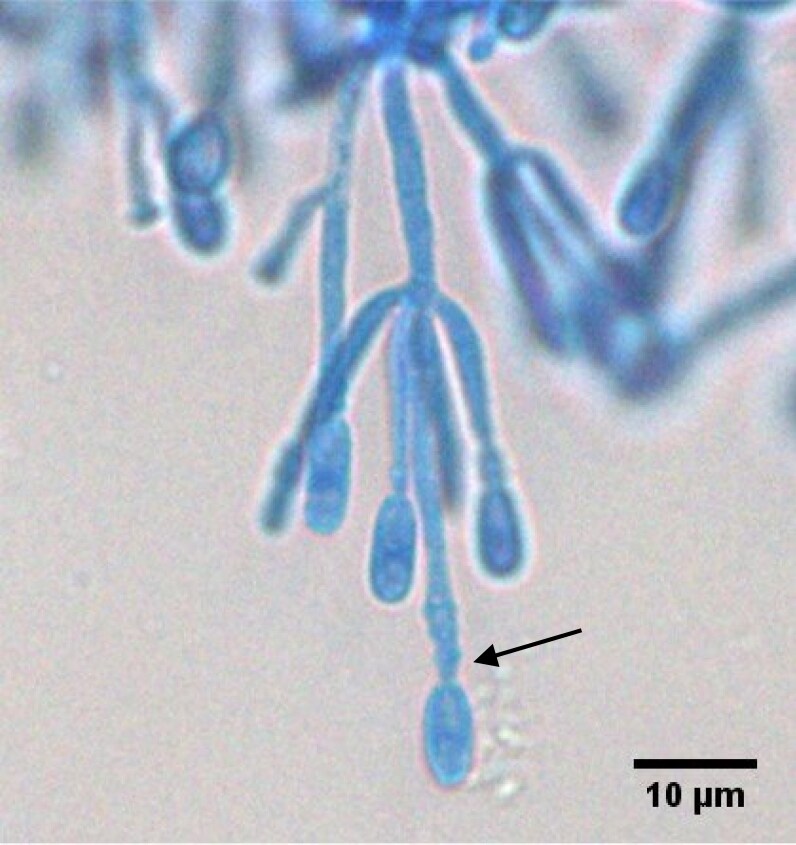
Banana- or canoe-shaped macroconidia of Fusarium solani can be observed forming on a short phialide/conidiophore (arrow). Prepared using adhesive tape method and stained using lactophenol blue. Courtesy of Professor Wieland Meyer, Ms Krystyna Maszewska, Dr Catriona Halliday, Mr Alex Khan, Mr Tsung-Yu Pai and Ms Georgia Clementine Wunderlich, Westerdijk Fungal Biodiversity Institute and Centre for Infectious Diseases and Microbiology, Westmead Hospital, Westmead, NSW, Australia.
Treatment
Treatment of infections due to Fusarium spp. is challenging due to their intrinsic resistance to many antifungal agents. Either voriconazole or L-AMB/amphotericin B lipid complex (5 mg/kg usually) are preferred as first-line treatment of fusariosis (Table 2).56,95–97 There are insufficient data to recommend one over the other. If a patient has refractory disease or is intolerant to one of voriconazole or L-AMB, then switching to the other antifungal agent is recommended (Table 2). If the patient is critically ill with fusariosis, then L-AMB and voriconazole can be used in combination until MIC results are available, then antifungal treatment can be rationalized (Table 2).96,98–101 Fusarium spp. generally have high MIC values to posaconazole. Posaconazole use should be guided by susceptibility testing and only used as salvage therapy, as modified-released tablets or IV formulations and with therapeutic drug monitoring.99,102–104 Table 2 gives a detailed description of all the recommended antifungal regimens. Fosmanogepix has in vitro activity against F. solani and F. oxysporum (MEC90 0.06 and 0.25–16 mg/L, respectively) (Table 3).112 Fosmanogepix was successfully used to treat patients who were part of the recent Mexican outbreak of Fusarium meningitis.167 Olorofim has variable activity against Fusarium spp. In the Phase II study (NCT03583164) olorofim could only be used if Fusarium spp. had low MIC values (Table 3).
Successful outcomes have been reported with surgery in cases of fusariosis involving the skin, bones and joints (Table 4).100,140 In cases with endophthalmitis, the involvement of an experienced ophthalmologist is strongly recommended. Intravitreal instillation of antifungal agents and vitrectomy may be required. Since voriconazole has good penetration into the eye we recommend this agent as first-line for intravitreal injections (Table 4). Like other NAMs, lines should be removed in the setting of fungaemia (if feasible). The use of G-CSF and granulocyte transfusions as adjuncts in the treatment of invasive fusariosis should be considered as they have been associated with a favourable outcomes of 41% and 91% (n = 11), respectively (Table 4).141,142 There are no data on the use of hyperbaric oxygen in cases of invasive fusariosis.
Other rare moulds
There are many other NAMs that rarely cause infection and if so it is mainly in the immunocompromised. A summary of the risk factors, diagnosis and treatment of these can be found in Table S1 (includes the related references) (available as Supplementary data at JAC Online). A more detailed description is beyond the scope of this review, but an excellent overview can be found in the global guidelines authored by Hoenigl et al.168
Conclusions
NAM infections are on the increase and are associated with high mortality rates. Hence, early diagnosis is critical to improve outcomes. To achieve this, clinicians need to have a high level of suspicion and a knowledge of the risk factors so that prompt investigation can be performed. Identification to species level is critical to guide antifungal therapy. Microscopy, culture and histology were the cornerstone of diagnosis, but molecular methods are becoming more important as new assays that can determine the fungus to species level are being developed. New antifungal therapies are being developed that have the potential to improve outcomes due to greater efficacy and less toxicity. Multidisciplinary care remains critical to optimizing outcomes. Ongoing improvements in the outcomes require a better understanding of the epidemiology and risk factors, the development of novel diagnostic tools and effective antifungal treatments, and a greater insight into the efficacy of the various adjunctive therapies.
Supplementary Material
Funding
This supplement was published as part of a supplement financially supported by F2G Ltd and Shionogi B.V.
Transparency declarations
C.O.M. has over the last 5 years received research grants from Gilead Sciences and Merck Sharp & Dohme Australia, and speaker’s honoraria from Gilead Sciences and Pfizer. All fees have been paid directly to Alfred Health.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Kontoyiannis DP, Marr KA, Park BJ et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 2. Park BJ, Pappas PG, Wannemuehler KA et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis 2011; 17: 1855–64. 10.3201/eid1710.110087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamaris GA, Chamilos G, Lewis RE et al. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin Infect Dis 2006; 43: 1580–4. 10.1086/509579 [DOI] [PubMed] [Google Scholar]
- 4. Dalyan Cilo B, Al-Hatmi AM, Seyedmousavi S et al. Emergence of fusarioses in a university hospital in Turkey during a 20-year period. Eur J Clin Microbiol Infect Dis 2015; 34: 1683–91. 10.1007/s10096-015-2405-y [DOI] [PubMed] [Google Scholar]
- 5. Lamoth F, Chung SJ, Damonti L et al. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis 2017; 64: 1619–21. 10.1093/cid/cix130 [DOI] [PubMed] [Google Scholar]
- 6. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis 2018; 66: 140–8. 10.1093/cid/cix687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis 2012; 54 Suppl 1: S8–15. 10.1093/cid/cir864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy KJ, Daveson K, Slavin MA et al. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect 2016; 22: 775–81. 10.1016/j.cmi.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 9. Lanternier F, Dannaoui E, Morizot G et al. A global analysis of mucormycosis in France: the RetroZygo study (2005-2007). Clin Infect Dis 2012; 54 Suppl 1: S35–43. 10.1093/cid/cir880 [DOI] [PubMed] [Google Scholar]
- 10. Skiada A, Pagano L, Groll A et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011; 17: 1859–67. 10.1111/j.1469-0691.2010.03456.x [DOI] [PubMed] [Google Scholar]
- 11. Rüping MJ, Heinz WJ, Kindo AJ et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother 2010; 65: 296–302. 10.1093/jac/dkp430 [DOI] [PubMed] [Google Scholar]
- 12. Ambrosioni J, Bouchuiguir-Wafa K, Garbino J. Emerging invasive zygomycosis in a tertiary care center: epidemiology and associated risk factors. Int J Infect Dis 2010; 14 Suppl 3: e100–3. 10.1016/j.ijid.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 13. Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses 2014; 57 Suppl 3: 85–90. 10.1111/myc.12243 [DOI] [PubMed] [Google Scholar]
- 14. Petrikkos G, Skiada A, Lortholary O et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012; 54 Suppl 1: S23–34. 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 15. Muthu V, Rudramurthy SM, Chakrabarti A et al. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 2021; 186: 739–54. 10.1007/s11046-021-00584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong W, Keighley C, Wolfe R et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019; 25: 26–34. 10.1016/j.cmi.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 17. Coste A, Conrad A, Porcher R et al. P397 influence of underlying conditions on disease presentation and diagnostic strategy during pulmonary mucormycosis: a national study of 114 cases. Med Mycol 2022; 60 Suppl 1: myac072P397. 10.1093/mmy/myac072.P397 [DOI] [Google Scholar]
- 18. Damaraju V, Agarwal R, Singh Sehgal I et al. Ibrutinib and tracheal mucormycosis: a case report and systematic review of literature. J Mycol Med 2023; 33: 101414. 10.1016/j.mycmed.2023.101414 [DOI] [PubMed] [Google Scholar]
- 19. Maggioni G, Fedrigo M, Visentin A et al. Severe fatal mucormycosis in a patient with chronic lymphocytic leukaemia treated with zanubrutinib: a case report and review of the literature. Curr Oncol 2023; 30: 8255–65. 10.3390/curroncol30090599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aboutanos MB, Joshi M, Scalea TM. Isolated pulmonary mucormycosis in a patient with multiple injuries: a case presentation and review of the literature. J Trauma 2003; 54: 1016–9. 10.1097/01.TA.0000023169.90650.6B [DOI] [PubMed] [Google Scholar]
- 21. Kontoyiannis DP, Azie N, Franks B et al. Prospective antifungal therapy (PATH) alliance(®): focus on mucormycosis. Mycoses 2014; 57: 240–6. 10.1111/myc.12149 [DOI] [PubMed] [Google Scholar]
- 22. Serris A, Danion F, Lanternier F. Disease entities in mucormycosis. J Fungi (Basel) 2019; 5: 23. 10.3390/jof5010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Addasi Y, Nguyen AH, Sabri A et al. Gastrointestinal mucormycosis: a clinical review. Gastroenterology Res 2023; 16: 249–53. 10.14740/gr1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pagano L, Ricci P, Tonso A et al. Mucormycosis in patients with haematological malignancies: a retrospective clinical study of 37 cases. GIMEMA infection program (Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto). Br J Haematol 1997; 99: 331–6. 10.1046/j.1365-2141.1997.3983214.x [DOI] [PubMed] [Google Scholar]
- 25. St-Germain G, Robert A, Ishak M et al. Infection due to Rhizomucor pusillus: report of four cases in patients with leukemia and review. Clin Infect Dis 1993; 16: 640–5. 10.1093/clind/16.5.640 [DOI] [PubMed] [Google Scholar]
- 26. Nosari A, Oreste P, Montillo M et al. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica 2000; 85: 1068–71. 10.3324/%x [DOI] [PubMed] [Google Scholar]
- 27. Kontoyiannis DP, Wessel VC, Bodey GP et al. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 2000; 30: 851–6. 10.1086/313803 [DOI] [PubMed] [Google Scholar]
- 28. Roden MM, Zaoutis TE, Buchanan WL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 29. Marchiori E, Zanetti G, Escuissato DL et al. Reversed halo sign: high-resolution CT scan findings in 79 patients. Chest 2012; 141: 1260–6. 10.1378/chest.11-1050 [DOI] [PubMed] [Google Scholar]
- 30. Sassi C, Stanzani M, Lewis RE et al. The utility of contrast-enhanced hypodense sign for the diagnosis of pulmonary invasive mould disease in patients with haematological malignancies. Br J Radiol 2018; 91: 20170220. 10.1259/bjr.20170220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect 2009; 15 Suppl 5: 60–5. 10.1111/j.1469-0691.2009.02999.x [DOI] [PubMed] [Google Scholar]
- 32. Rychert J, Slechta ES, Barker AP et al. Multicenter evaluation of the Vitek MS v3.0 system for the identification of filamentous fungi. J Clin Microbiol 2018; 56: e01353-17. 10.1128/JCM.01353-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamoth F, Kontoyiannis DP. PCR diagnostic platforms for non-Aspergillus mold infections: ready for routine implementation in the clinic? Expert Rev Mol Diagn 2024; 24: 273–82. 10.1080/14737159.2024.2326474 [DOI] [PubMed] [Google Scholar]
- 34. Millon L, Larosa F, Lepiller Q et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 2013; 56: e95–101. 10.1093/cid/cit094 [DOI] [PubMed] [Google Scholar]
- 35. Millon L, Herbrecht R, Grenouillet F et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 2016; 22: 810.e1–e8. 10.1016/j.cmi.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 36. Lamoth F, Damonti L, Alexander BD. Role of antifungal susceptibility testing in non-Aspergillus invasive mold infections. J Clin Microbiol 2016; 54: 1638–40. 10.1128/JCM.00318-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guinea J, Peláez T, Recio S et al. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 2008; 52: 1396–400. 10.1128/AAC.01512-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halliday CL, Chen SC, Kidd SE et al. Antifungal susceptibilities of non-Aspergillus filamentous fungi causing invasive infection in Australia: support for current antifungal guideline recommendations. Int J Antimicrob Agents 2016; 48: 453–8. 10.1016/j.ijantimicag.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 39. Lamoth F, Nucci M, Fernandez-Cruz A et al. Performance of the beta-glucan test for the diagnosis of invasive fusariosis and scedosporiosis: a meta-analysis. Med Mycol 2023; 61: myad061. 10.1093/mmy/myad061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hedayati MT, Tavakoli M, Maleki M et al. Fungal epidemiology in cystic fibrosis patients with a special focus on Scedosporium species complex. Microb Pathog 2019; 129: 168–75. 10.1016/j.micpath.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 41. Lau A, Chen S, Sorrell T et al. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J Clin Microbiol 2007; 45: 380–5. 10.1128/JCM.01862-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rougeron A, Giraud S, Alastruey-Izquierdo A et al. Ecology of Scedosporium species: present knowledge and future research. Mycopathologia 2018; 183: 185–200. 10.1007/s11046-017-0200-2 [DOI] [PubMed] [Google Scholar]
- 43. Lackner M, de Hoog GS, Verweij PE et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 2012; 56: 2635–42. 10.1128/AAC.05910-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lackner M, Hagen F, Meis JF et al. Susceptibility and diversity in the therapy-refractory genus Scedosporium. Antimicrob Agents Chemother 2014; 58: 5877–85. 10.1128/AAC.03211-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rodriguez-Tudela JL, Berenguer J, Guarro J et al. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol 2009; 47: 359–70. 10.1080/13693780802524506 [DOI] [PubMed] [Google Scholar]
- 46. Heath CH, Slavin MA, Sorrell TC et al. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin Microbiol Infect 2009; 15: 689–93. 10.1111/j.1469-0691.2009.02802.x [DOI] [PubMed] [Google Scholar]
- 47. Bouchara JP, Hsieh HY, Croquefer S et al. Development of an oligonucleotide array for direct detection of fungi in sputum samples from patients with cystic fibrosis. J Clin Microbiol 2009; 47: 142–52. 10.1128/JCM.01668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boch T, Reinwald M, Postina P et al. Identification of invasive fungal diseases in immunocompromised patients by combining an Aspergillus specific PCR with a multifungal DNA-microarray from primary clinical samples. Mycoses 2015; 58: 735–45. 10.1111/myc.12424 [DOI] [PubMed] [Google Scholar]
- 49. Lu Q, van den Ende AH, de Hoog GS et al. Reverse line blot hybridisation screening of Pseudallescheria/Scedosporium species in patients with cystic fibrosis. Mycoses 2011; 54 Suppl 3: 5–11. 10.1111/j.1439-0507.2011.02108.x [DOI] [PubMed] [Google Scholar]
- 50. Lau A, Sorrell TC, Chen S et al. Multiplex tandem PCR: a novel platform for rapid detection and identification of fungal pathogens from blood culture specimens. J Clin Microbiol 2008; 46: 3021–7. 10.1128/JCM.00689-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spiess B, Seifarth W, Hummel M et al. DNA microarray-based detection and identification of fungal pathogens in clinical samples from neutropenic patients. J Clin Microbiol 2007; 45: 3743–53. 10.1128/JCM.00942-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu Q, Gerrits van den Ende AH, Bakkers JM et al. Identification of Pseudallescheria and Scedosporium species by three molecular methods. J Clin Microbiol 2011; 49: 960–7. 10.1128/JCM.01813-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harun A, Blyth CC, Gilgado F et al. Development and validation of a multiplex PCR for detection of Scedosporium spp. in respiratory tract specimens from patients with cystic fibrosis. J Clin Microbiol 2011; 49: 1508–12. 10.1128/JCM.01810-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nucci M, Barreiros G, Akiti T et al. Invasive fusariosis in patients with hematologic diseases. J Fungi (Basel) 2021; 7: 815. 10.3390/jof7100815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nucci F, Nouér SA, Capone D et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect 2018; 24: 1105.e1–.e4. 10.1016/j.cmi.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 56. Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 2007; 20: 695–704. 10.1128/CMR.00014-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Triest D, Stubbe D, De Cremer K et al. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of molds of the Fusarium genus. J Clin Microbiol 2015; 53: 465–76. 10.1128/JCM.02213-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nucci M, Carlesse F, Cappellano P et al. Earlier diagnosis of invasive fusariosis with Aspergillus serum galactomannan testing. PLoS One 2014; 9: e87784. 10.1371/journal.pone.0087784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nucci M, Barreiros G, Reis H et al. Performance of 1,3-beta-D-glucan in the diagnosis and monitoring of invasive fusariosis. Mycoses 2019; 62: 570–5. 10.1111/myc.12918 [DOI] [PubMed] [Google Scholar]
- 60. de Souza M, Matsuzawa T, Sakai K et al. Comparison of DNA microarray, loop-mediated isothermal amplification (LAMP) and real-time PCR with DNA sequencing for identification of Fusarium spp. obtained from patients with hematologic malignancies. Mycopathologia 2017; 182: 625–32. 10.1007/s11046-017-0129-5 [DOI] [PubMed] [Google Scholar]
- 61. Al-Hatmi AM, Bonifaz A, Tirado-Sánchez A et al. Fusarium species causing eumycetoma: report of two cases and comprehensive review of the literature. Mycoses 2017; 60: 204–12. 10.1111/myc.12590 [DOI] [PubMed] [Google Scholar]
- 62. Landlinger C, Preuner S, Willinger B et al. Species-specific identification of a wide range of clinically relevant fungal pathogens by use of luminex xMAP technology. J Clin Microbiol 2009; 47: 1063–73. 10.1128/JCM.01558-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Landlinger C, Preuner S, Bašková L et al. Diagnosis of invasive fungal infections by a real-time panfungal PCR assay in immunocompromised pediatric patients. Leukemia 2010; 24: 2032–8. 10.1038/leu.2010.209 [DOI] [PubMed] [Google Scholar]
- 64. Balajee SA, Borman AM, Brandt ME et al. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J Clin Microbiol 2009; 47: 877–84. 10.1128/JCM.01685-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al-Hatmi AMS, Bonifaz A, Ranque S et al. Current antifungal treatment of fusariosis. Int J Antimicrob Agents 2018; 51: 326–32. 10.1016/j.ijantimicag.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 66. Espinel-Ingroff A, Colombo AL, Cordoba S et al. International evaluation of MIC distributions and epidemiological cutoff value (ECV) definitions for Fusarium species identified by molecular methods for the CLSI broth microdilution method. Antimicrob Agents Chemother 2016; 60: 1079–84. 10.1128/AAC.02456-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Herrera DA, Dublin AB, Ormsby EL et al. Imaging findings of rhinocerebral mucormycosis. Skull Base 2009; 19: 117–25. 10.1055/s-0028-1096209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jensen HE, Salonen J, Ekfors TO. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J Pathol 1997; 181: 100–5. [DOI] [PubMed] [Google Scholar]
- 69. Bupha-Intr O, Butters C, Reynolds G et al. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern Med J 2021; 51 Suppl 7: 177–219. 10.1111/imj.15592 [DOI] [PubMed] [Google Scholar]
- 70. Chau MM, Daveson K, Alffenaar JC et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy and haemopoietic stem cell transplant recipients, 2021. Intern Med J 2021; 51 Suppl 7: 37–66. 10.1111/imj.15587 [DOI] [PubMed] [Google Scholar]
- 71. Andes D, Kovanda L, Desai A et al. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 2018; 62: e00585-18. 10.1128/AAC.00585-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ibrahim AS, Avanessian V, Spellberg B et al. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother 2003; 47: 3343–4. 10.1128/AAC.47.10.3343-3344.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walsh TJ, Goodman JL, Pappas P et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother 2001; 45: 3487–96. 10.1128/AAC.45.12.3487-3496.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lanternier F, Poiree S, Elie C et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother 2015; 70: 3116–23. 10.1093/jac/dkv236 [DOI] [PubMed] [Google Scholar]
- 75. Salmanton-García J, Seidel D, Koehler P et al. Matched-paired analysis of patients treated for invasive mucormycosis: standard treatment versus posaconazole new formulations (MoveOn). J Antimicrob Chemother 2019; 74: 3315–27. 10.1093/jac/dkz344 [DOI] [PubMed] [Google Scholar]
- 76. Marty FM, Ostrosky-Zeichner L, Cornely OA et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828–37. 10.1016/S1473-3099(16)00071-2 [DOI] [PubMed] [Google Scholar]
- 77. Groll AH, Giri N, Petraitis V et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 2000; 182: 274–82. 10.1086/315643 [DOI] [PubMed] [Google Scholar]
- 78. Ibrahim AS, Gebremariam T, Fu Y et al. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother 2008; 52: 1556–8. 10.1128/AAC.01458-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Spellberg B, Fu Y, Edwards JE Jr et al. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother 2005; 49: 830–2. 10.1128/AAC.49.2.830-832.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reed C, Bryant R, Ibrahim AS et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008; 47: 364–71. 10.1086/589857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pagano L, Cornely OA, Busca A et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: a report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013; 98: e127–30. 10.3324/haematol.2012.083063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Husain S, Muñoz P, Forrest G et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 2005; 40: 89–99. 10.1086/426445 [DOI] [PubMed] [Google Scholar]
- 83. Seidel D, Meißner A, Lackner M et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit Rev Microbiol 2019; 45: 1–21. 10.1080/1040841X.2018.1514366 [DOI] [PubMed] [Google Scholar]
- 84. Alastruey-Izquierdo A, Alcazar-Fuoli L, Rivero-Menéndez O et al. Molecular identification and susceptibility testing of molds isolated in a prospective surveillance of triazole resistance in Spain (FILPOP2 study). Antimicrob Agents Chemother 2018; 62: e00358-18. 10.1128/AAC.00358-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meletiadis J, Mouton JW, Meis JF et al. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother 2003; 47: 106–17. 10.1128/AAC.47.1.106-117.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dolton MJ, Perera V, Pont LG et al. Terbinafine in combination with other antifungal agents for treatment of resistant or refractory mycoses: investigating optimal dosing regimens using a physiologically based pharmacokinetic model. Antimicrob Agents Chemother 2014; 58: 48–54. 10.1128/AAC.02006-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jenks JD, Reed SL, Seidel D et al. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: the role of antifungal combination therapy. Int J Antimicrob Agents 2018; 52: 706–12. 10.1016/j.ijantimicag.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Perfect JR, Marr KA, Walsh TJ et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis 2003; 36: 1122–31. 10.1086/374557 [DOI] [PubMed] [Google Scholar]
- 89. Goldman C, Akiyama MJ, Torres J et al. Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: a case report and review of the literature. Med Mycol Case Rep 2016; 11: 40–3. 10.1016/j.mmcr.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mellinghoff IK, Winston DJ, Mukwaya G et al. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin Infect Dis 2002; 34: 1648–50. 10.1086/340522 [DOI] [PubMed] [Google Scholar]
- 91. Jenks JD, Seidel D, Cornely OA et al. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: analysis of 41 patients from the FungiScope® registry 2008-2019. Clin Microbiol Infect 2020; 26: 784.e1–e5. 10.1016/j.cmi.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 92. Howden BP, Slavin MA, Schwarer AP et al. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur J Clin Microbiol Infect Dis 2003; 22: 111–3. 10.1007/s10096-002-0877-z [DOI] [PubMed] [Google Scholar]
- 93. Cobo F, Lara-Oya A, Rodríguez-Granger J et al. Infections caused by Scedosporium/Lomentospora species: clinical and microbiological findings in 21 cases. Med Mycol 2018; 56: 917–25. 10.1093/mmy/myx147 [DOI] [PubMed] [Google Scholar]
- 94. Troke P, Aguirrebengoa K, Arteaga C et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother 2008; 52: 1743–50. 10.1128/AAC.01388-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taj-Aldeen SJ. Reduced multidrug susceptibility profile is a common feature of opportunistic Fusarium species: fusarium multi-drug resistant pattern. J Fungi (Basel) 2017; 3: 18. 10.3390/jof3020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nucci M, Marr KA, Vehreschild MJ et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect 2014; 20: 580–5. 10.1111/1469-0691.12409 [DOI] [PubMed] [Google Scholar]
- 97. Tortorano AM, Prigitano A, Esposto MC et al. European confederation of medical mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis 2014; 33: 1623–30. 10.1007/s10096-014-2111-1 [DOI] [PubMed] [Google Scholar]
- 98. Stempel JM, Hammond SP, Sutton DA et al. Invasive fusariosis in the voriconazole era: single-center 13-year experience. Open Forum Infect Dis 2015; 2: ofv099. 10.1093/ofid/ofv099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Horn DL, Freifeld AG, Schuster MG et al. Treatment and outcomes of invasive fusariosis: review of 65 cases from the PATH alliance(®) registry. Mycoses 2014; 57: 652–8. 10.1111/myc.12212 [DOI] [PubMed] [Google Scholar]
- 100. Muhammed M, Anagnostou T, Desalermos A et al. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore) 2013; 92: 305–16. 10.1097/MD.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lortholary O, Obenga G, Biswas P et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother 2010; 54: 4446–50. 10.1128/AAC.00286-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Campo M, Lewis RE, Kontoyiannis DP. Invasive fusariosis in patients with hematologic malignancies at a cancer center: 1998-2009. J Infect 2010; 60: 331–7. 10.1016/j.jinf.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 103. Raad II, Graybill JR, Bustamante AB et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis 2006; 42: 1726–34. 10.1086/504328 [DOI] [PubMed] [Google Scholar]
- 104. Lewis RE, Albert ND, Kontoyiannis DP. Comparative pharmacodynamics of posaconazole in neutropenic murine models of invasive pulmonary aspergillosis and mucormycosis. Antimicrob Agents Chemother 2014; 58: 6767–72. 10.1128/AAC.03569-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother 2017; 72: i12–i8. 10.1093/jac/dkx029 [DOI] [PubMed] [Google Scholar]
- 106. Miceli MH, Kauffman CA. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 2015; 61: 1558–65. 10.1093/cid/civ571 [DOI] [PubMed] [Google Scholar]
- 107. Maertens JA, Rahav G, Lee DG et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet 2021; 397: 499–509. 10.1016/S0140-6736(21)00219-1 [DOI] [PubMed] [Google Scholar]
- 108. Ullmann AJ, Cornely OA, Burchardt A et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother 2006; 50: 658–66. 10.1128/AAC.50.2.658-666.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gebremariam T, Alkhazraji S, Alqarihi A et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother 2020; 64: e00178-20. 10.1128/AAC.00178-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pfaller MA, Huband MD, Flamm RK et al. In vitro activity of APX001A (Manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother 2019; 63: e00840-19. 10.1128/AAC.00840-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J Antimicrob Chemother 2019; 74: 1295–9. 10.1093/jac/dkz022 [DOI] [PubMed] [Google Scholar]
- 112. Castanheira M, Duncanson FP, Diekema DJ et al. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother 2012; 56: 352–7. 10.1128/AAC.05414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gebremariam T, Alkhazraji S, Alqarihi A et al. APX001A protects immunosuppressed mice from Rhizopus delemar infection. Open Forum Infect Dis 2017; 4: S475. 10.1093/ofid/ofx163.1217 [DOI] [Google Scholar]
- 114. Alkhazraji S, Gebremariam T, Alqarihi A et al. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother 2020; 64: e01735-19. 10.1128/AAC.01735-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of olorofim against clinical isolates of Scedosporium species and Lomentospora prolificans using EUCAST and CLSI methodologies. J Antimicrob Chemother 2020; 75: 3582–5. 10.1093/jac/dkaa351 [DOI] [PubMed] [Google Scholar]
- 116. Seyedmousavi S, Chang YC, Youn JH et al. In vivo efficacy of olorofim against systemic scedosporiosis and lomentosporiosis. Antimicrob Agents Chemother 2021; 65: e0043421. 10.1128/AAC.00434-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Georgacopoulos O, Nunnally NS, Ransom EM et al. In vitro activity of novel antifungal olorofim against filamentous fungi and comparison to eight other antifungal agents. J Fungi (Basel) 2021; 7: 378. 10.3390/jof7050378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lamoth F, Alexander BD. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 2015; 59: 4308–11. 10.1128/AAC.00234-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gebremariam T, Alkhazraji S, Gu Y et al. Ibrexafungerp is efficacious in a neutropenic murine model of pulmonary mucormycosis as monotherapy and combined with liposomal amphotericin B. Antimicrob Agents Chemother 2024; 68: e0154523. 10.1128/aac.01545-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gebremariam T, Wiederhold NP, Fothergill AW et al. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob Agents Chemother 2015; 59: 7815–7. 10.1128/AAC.01437-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wiederhold NP, Patterson HP, Tran BH et al. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J Antimicrob Chemother 2018; 73: 404–8. 10.1093/jac/dkx410 [DOI] [PubMed] [Google Scholar]
- 122. Rauseo AM, Coler-Reilly A, Larson L et al. Hope on the horizon: novel fungal treatments in development. Open Forum Infect Dis 2020; 7: ofaa016. 10.1093/ofid/ofaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pfaller MA, Huband MD, Flamm RK et al. Antimicrobial activity of manogepix, a first-in-class antifungal, and comparator agents tested against contemporary invasive fungal isolates from an international surveillance programme (2018-2019). J Glob Antimicrob Resist 2021; 26: 117–27. 10.1016/j.jgar.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 124. Greenberg RN, Mullane K, van Burik JA et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother 2006; 50: 126–33. 10.1128/AAC.50.1.126-133.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Taj-Aldeen SJ, Gamaletsou MN, Rammaert B et al. Bone and joint infections caused by mucormycetes: a challenging osteoarticular mycosis of the twenty-first century. Med Mycol 2017; 55: 691–704. 10.1093/mmy/myw136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Saxena P, Shen SH, Morrissey O et al. Challenges in the management of invasive pulmonary zygomycosis: the Alfred experience. ANZ J Surg 2015; 85: 700–1. 10.1111/ans.13264 [DOI] [PubMed] [Google Scholar]
- 127. Janum S, Afshari A. Central venous catheter (CVC) removal for patients of all ages with candidaemia. Cochrane Database Syst Rev 2016; 7: CD011195. 10.1002/14651858.CD011195.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Farina C, Marchesi G, Passera M et al. In vitro activity of amphotericin B against zygomycetes isolated from deep mycoses: a comparative study between incubation in aerobic and hyperbaric atmosphere. Med Mycol 2012; 50: 427–32. 10.3109/13693786.2011.614964 [DOI] [PubMed] [Google Scholar]
- 129. John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect 2005; 11: 515–7. 10.1111/j.1469-0691.2005.01170.x [DOI] [PubMed] [Google Scholar]
- 130. Ferguson BJ, Mitchell TG, Moon R et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis 1988; 10: 551–9. 10.1093/clinids/10.3.551 [DOI] [PubMed] [Google Scholar]
- 131. Ribeiro EF, dos Santos VM, Paixão GT et al. Mucormycosis in a patient with acute myeloid leukemia successfully treated with liposomal amphotericin B associated with deferasirox and hyperbaric oxygen. Mycopathologia 2013; 175: 295–300. 10.1007/s11046-013-9629-0 [DOI] [PubMed] [Google Scholar]
- 132. Gebremariam T, Lin L, Liu M et al. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest 2016; 126: 2280–94. 10.1172/JCI82744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu M, Spellberg B, Phan QT et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest 2010; 120: 1914–24. 10.1172/JCI42164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Afroze SN, Korlepara R, Rao GV et al. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent 2017; 8: 662–6. 10.4103/ccd.ccd_558_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schmidt S, Tramsen L, Perkhofer S et al. Characterization of the cellular immune responses to Rhizopus oryzae with potential impact on immunotherapeutic strategies in hematopoietic stem cell transplantation. J Infect Dis 2012; 206: 135–9. 10.1093/infdis/jis308 [DOI] [PubMed] [Google Scholar]
- 136. Gil-Lamaignere C, Winn RM, Simitsopoulou M et al. Interferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparison with Aspergillus spp. Med Mycol 2005; 43: 253–60. 10.1080/13693780412331271072 [DOI] [PubMed] [Google Scholar]
- 137. Ortoneda M, Capilla J, Pujol I et al. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J Antimicrob Chemother 2002; 49: 525–9. 10.1093/jac/49.3.525 [DOI] [PubMed] [Google Scholar]
- 138. Grenouillet F, Botterel F, Crouzet J et al. Scedosporium prolificans: an emerging pathogen in France? Med Mycol 2009; 47: 343–50. 10.1080/13693780802454761 [DOI] [PubMed] [Google Scholar]
- 139. Simitsopoulou M, Gil-Lamaignere C, Avramidis N et al. Antifungal activities of posaconazole and granulocyte-macrophage colony-stimulating factor ex vivo and in mice with disseminated infection due to Scedosporium prolificans. Antimicrob Agents Chemother 2004; 48: 3801–5. 10.1128/AAC.48.10.3801-3805.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Koehler P, Tacke D, Cornely OA. Bone and joint infections by Mucorales, Scedosporium, Fusarium and even rarer fungi. Crit Rev Microbiol 2016; 42: 158–71. 10.3109/1040841X.2014.910749 [DOI] [PubMed] [Google Scholar]
- 141. Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 1997; 90: 999–1008. 10.1182/blood.V90.3.999 [DOI] [PubMed] [Google Scholar]
- 142. Rizzello I, Castagnetti F, Toschi PG et al. Successful treatment of bilateral endogenous Fusarium solani endophthalmitis in a patient with acute lymphocytic leukaemia. Mycoses 2018; 61: 53–60. 10.1111/myc.12697 [DOI] [PubMed] [Google Scholar]
- 143. Wurster S, Albert ND, Bharadwaj U et al. Blockade of the PD-1/PD-L1 immune checkpoint pathway improves infection outcomes and enhances fungicidal host defense in a murine model of invasive pulmonary mucormycosis. Front Immunol 2022; 13: 838344. 10.3389/fimmu.2022.838344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Serris A, Ouedrani A, Uhel F et al. Case report: immune checkpoint blockade plus interferon-Γ add-on antifungal therapy in the treatment of refractory COVID-associated pulmonary aspergillosis and cerebral mucormycosis. Front Immunol 2022; 13: 900522. 10.3389/fimmu.2022.900522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gebremariam T, Alkhazraji S, Soliman SSM et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci Adv 2019; 5: eaaw1327. 10.1126/sciadv.aaw1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Alqarihi A, Gebremariam T, Gu Y et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio 2020; 11: e01087-20. 10.1128/mBio.01087-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kaltseis J, Rainer J, De Hoog GS et al. Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Med Mycol 2009; 47: 398–405. 10.1080/13693780802585317 [DOI] [PubMed] [Google Scholar]
- 148. Cortez KJ, Roilides E, Quiroz-Telles F et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 2008; 21: 157–97. 10.1128/CMR.00039-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ramirez-Garcia A, Pellon A, Rementeria A et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol 2018; 56 Suppl 1: 102–25. 10.1093/mmy/myx113 [DOI] [PubMed] [Google Scholar]
- 150. Maertens JA, Verweij PE, Lanuza EF et al. Olorofim for the treatment of invasive mould infections in patients with limited or no treatment options: comparison of interim results from a phase 2B open-label study with outcomes in historical control populations (NCT03583164, FORMULA-OLS, study 32). Open Forum Infect Dis 2022; 9 Suppl 2: ofac492-063. 10.1093/ofid/ofac492.063. [DOI] [Google Scholar]
- 151. Lackner M, de Hoog GS, Yang L et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 2014; 67: 1–10. 10.1007/s13225-014-0295-4 [DOI] [Google Scholar]
- 152. Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev 2010; 23: 884–928. 10.1128/CMR.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Penteado FD, Litvinov N, Sztajnbok J et al. Lomentospora prolificans fungemia in hematopoietic stem cell transplant patients: first report in South America and literature review. Transpl Infect Dis 2018; 20: e12908. 10.1111/tid.12908 [DOI] [PubMed] [Google Scholar]
- 154. Tong SY, Peleg AY, Yoong J et al. Breakthrough Scedosporium prolificans infection while receiving voriconazole prophylaxis in an allogeneic stem cell transplant recipient. Transpl Infect Dis 2007; 9: 241–3. 10.1111/j.1399-3062.2007.00203.x [DOI] [PubMed] [Google Scholar]
- 155. O'Donnell K, Sutton DA, Rinaldi MG et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 2010; 48: 3708–18. 10.1128/JCM.00989-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. van Diepeningen AD, Feng P, Ahmed S et al. Spectrum of Fusarium infections in tropical dermatology evidenced by multilocus sequencing typing diagnostics. Mycoses 2015; 58: 48–57. 10.1111/myc.12273 [DOI] [PubMed] [Google Scholar]
- 157. Al-Hatmi AM, Hagen F, Menken SB et al. Global molecular epidemiology and genetic diversity of Fusarium, a significant emerging group of human opportunists from 1958 to 2015. Emerg Microbes Infect 2016; 5: e124. 10.1038/emi.2016.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rosa PDD, Ramirez-Castrillon M, Borges R et al. Epidemiological aspects and characterization of the resistance profile of Fusarium spp. in patients with invasive fusariosis. J Med Microbiol 2019; 68: 1489–96. 10.1099/jmm.0.001059 [DOI] [PubMed] [Google Scholar]
- 159. Riches ML, Trifilio S, Chen M et al. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: a CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant 2016; 51: 277–82. 10.1038/bmt.2015.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nucci M, Marr KA, Queiroz-Telles F et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis 2004; 38: 1237–42. 10.1086/383319 [DOI] [PubMed] [Google Scholar]
- 161. Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis 2002; 35: 909–20. 10.1086/342328 [DOI] [PubMed] [Google Scholar]
- 162. Liu K, Howell DN, Perfect JR et al. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol 1998; 109: 45–54. 10.1093/ajcp/109.1.45 [DOI] [PubMed] [Google Scholar]
- 163. Healy M, Reece K, Walton D et al. Use of the Diversi lab system for species and strain differentiation of Fusarium species isolates. J Clin Microbiol 2005; 43: 5278–80. 10.1128/JCM.43.10.5278-5280.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Thomas B, Contet Audonneau N, Machouart M et al. Molecular identification of Fusarium species complexes: which gene and which database to choose in clinical practice? J Mycol Med 2019; 29: 56–8. 10.1016/j.mycmed.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 165. O'Donnell K, Sutton DA, Rinaldi MG et al. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J Clin Microbiol 2004; 42: 5109–20. 10.1128/JCM.42.11.5109-5120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Herkert PF, Al-Hatmi AMS, de Oliveira Salvador GL et al. Molecular characterization and antifungal susceptibility of clinical Fusarium species from Brazil. Front Microbiol 2019; 10: 737. 10.3389/fmicb.2019.00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Smith DJ, Gold JAW, Chiller T et al. Update on outbreak of fungal meningitis among US residents who received epidural anesthesia at two clinics in Matamoros, Mexico. Clin Infect Dis 2024; 78: 1554–8. 10.1093/cid/ciad570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hoenigl M, Salmanton-García J, Walsh TJ et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021; 21: e246–57. 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.