Abstract
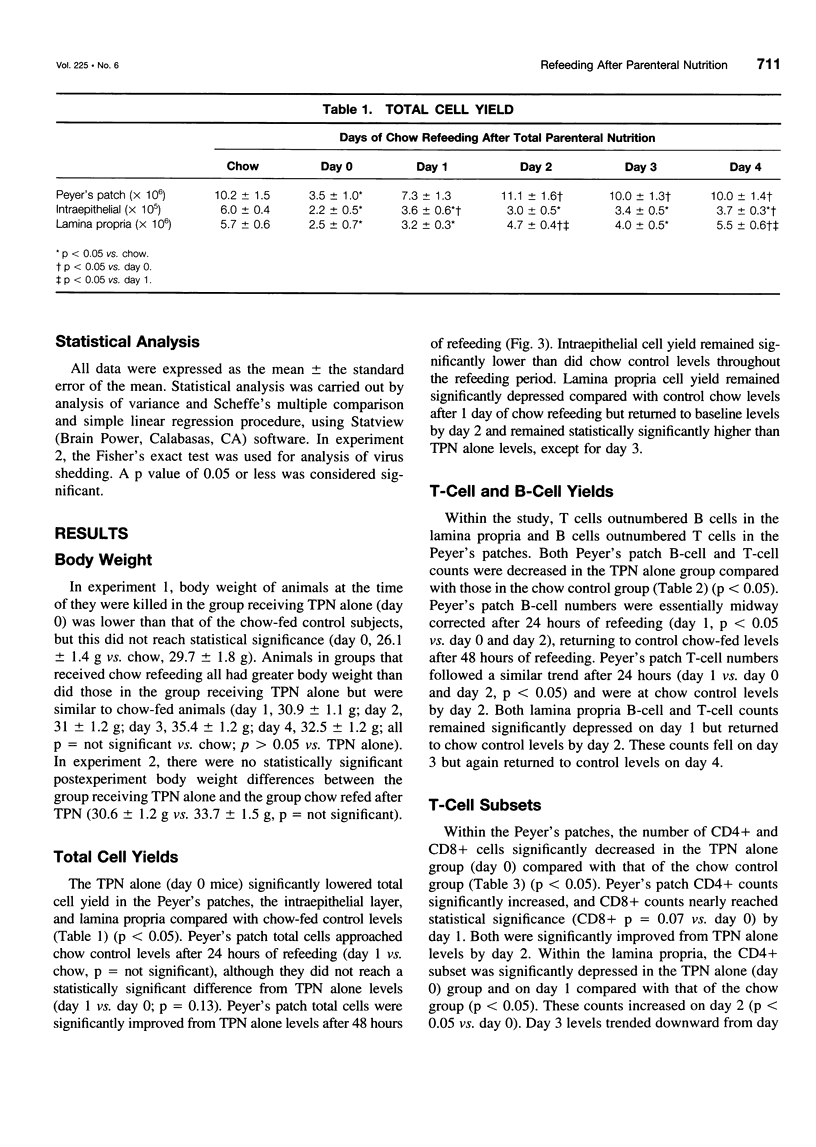
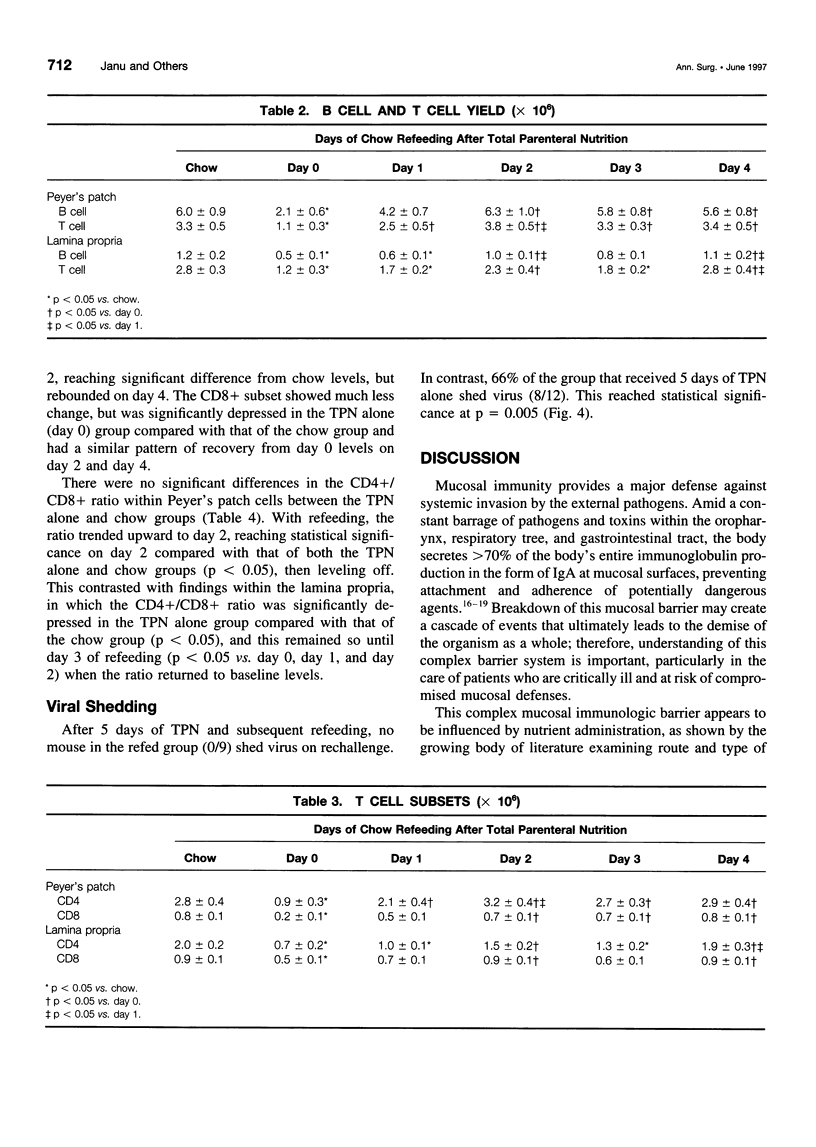
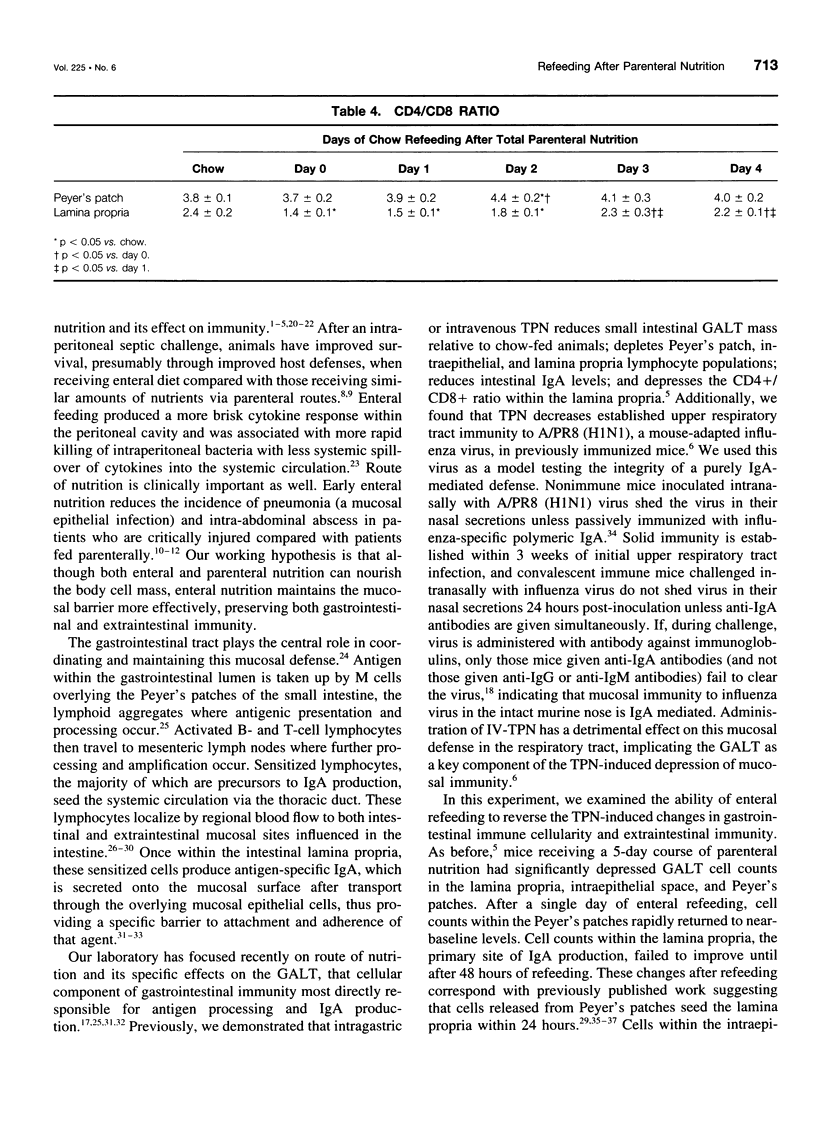
OBJECTIVE: The authors characterize the recovery of parenteral nutrition-induced changes in gut-associated lymphoid tissue (GALT) and upper respiratory tract immunity with enteral nutrition and provide further information defining the effects of enteral feeding on mucosal immunity. SUMMARY BACKGROUND DATA: The small intestine plays a prominent role in development and maintenance of mucosal immunity, both intestinal and extraintestinal, primarily through immunoglobulin A (IgA)-mediated mechanisms. Prior research has shown that mice fed total parenteral nutrition (TPN) have reduced GALT T and B cells, the cells responsible for IgA production, as well as impaired upper respiratory tract immunity to viral challenge of previously immunized animals. The recovery of TPN-induced changes in GALT and upper respiratory tract immunity after enteral refeeding is studied. METHODS: Male institute of Cancer Research mice received 5 days of TPN followed by 0 to 4 days of chow. Small intestinal GALT was characterized by flow cytometry. In a second experiment, animals were immunized intranasally with moused-adapted influenza virus. Three weeks later, one group received a 5-day course of TPN followed by enteral refeeding for 5 days. A second group received TPN alone. Both groups were challenged with intranasal virus and killed 40 hours postchallenge to determine viral shedding from the upper respiratory tract. RESULTS: Animals fed TPN only had significantly fewer GALT lymphocytes compared with those chow-fed control subjects. Peyer's patch counts increased after a single day of refeeding, returning to normal levels by 48 hours. Lamina propria counts remained significantly depressed after 24 hours of refeeding, but also returned to normal after 48 hours of refeeding. The T-cell and B-cell populations mimicked total cell patterns. Lamina propria CD4+/CD8+ ratio returned to normal only after 72 hours of refeeding. None of the 9 animals refed enterally for 5 days were positive for viral shedding, compared with 8 of 12 matched TPN-fed animals. CONCLUSIONS: Enteral refeeding after TPN is associated with rapid repletion of GALT cellularity, initially within Peyer's patches and subsequently within the lamina propria. Refeeding corrects the impairment of IgA-mediated upper respiratory tract antiviral immunity occurring with TPN administration. This work further enhances the authors' knowledge of the underlying immunologic differences influenced by routes of nutrition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alverdy J. C., Aoys E., Moss G. S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988 Aug;104(2):185–190. [PubMed] [Google Scholar]
- Barry W. S., Pierce N. F. Protein deprivation causes reversible impariment of mucosal immune response to cholera toxoid/toxin in rat gut. Nature. 1979 Sep 6;281(5726):64–65. doi: 10.1038/281064a0. [DOI] [PubMed] [Google Scholar]
- Barton R. G., Wells C. L., Carlson A., Singh R., Sullivan J. J., Cerra F. B. Dietary omega-3 fatty acids decrease mortality and Kupffer cell prostaglandin E2 production in a rat model of chronic sepsis. J Trauma. 1991 Jun;31(6):768–774. doi: 10.1097/00005373-199106000-00006. [DOI] [PubMed] [Google Scholar]
- Buchman A. L., Moukarzel A. A., Bhuta S., Belle M., Ament M. E., Eckhert C. D., Hollander D., Gornbein J., Kopple J. D., Vijayaroghavan S. R. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995 Nov-Dec;19(6):453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Li M., Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987 Jun;205(6):681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Xu D. Z., Qi L. Different lymphocyte compartments respond differently to mitogenic stimulation after thermal injury. Ann Surg. 1990 Jan;211(1):72–77. doi: 10.1097/00000658-199001000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Xu D., Qi L., Berg R. Elemental diet-induced immune suppression is caused by both bacterial and dietary factors. JPEN J Parenter Enteral Nutr. 1993 Jul-Aug;17(4):332–336. doi: 10.1177/0148607193017004332. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janu P. G., Kudsk K. A., Li J., Renegar K. B. Effect of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg. 1997 Jan;132(1):89–93. doi: 10.1001/archsurg.1997.01430250091019. [DOI] [PubMed] [Google Scholar]
- Kudsk K. A., Carpenter G., Petersen S., Sheldon G. F. Effect of enteral and parenteral feeding in malnourished rats with E. coli-hemoglobin adjuvant peritonitis. J Surg Res. 1981 Aug;31(2):105–110. doi: 10.1016/0022-4804(81)90037-8. [DOI] [PubMed] [Google Scholar]
- Kudsk K. A., Croce M. A., Fabian T. C., Minard G., Tolley E. A., Poret H. A., Kuhl M. R., Brown R. O. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992 May;215(5):503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk K. A., Li J., Renegar K. B. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996 Jun;223(6):629–638. doi: 10.1097/00000658-199606000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk K. A., Stone J. M., Carpenter G., Sheldon G. F. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983 Jul;23(7):605–609. doi: 10.1097/00005373-198307000-00010. [DOI] [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Li J., Kudsk K. A., Gocinski B., Dent D., Glezer J., Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995 Jul;39(1):44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Saito H., Fukushima R., Inaba T., Fukatsu K., Inoue T., Furukawa S., Han I., Muto T. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg. 1996 Jan;223(1):84–93. doi: 10.1097/00000658-199601000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous M., Xu D. Z., Lu Q., Berg R. D., Deitch E. A. Oral-TPN-induced bacterial translocation and impaired immune defenses are reversed by refeeding. Surgery. 1991 Aug;110(2):277–284. [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Moore F. A., Feliciano D. V., Andrassy R. J., McArdle A. H., Booth F. V., Morgenstein-Wagner T. B., Kellum J. M., Jr, Welling R. E., Moore E. E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992 Aug;216(2):172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F. A., Moore E. E., Jones T. N., McCroskey B. L., Peterson V. M. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989 Jul;29(7):916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Bruce R. G., Parrott D. M. The in-vivo kinetics of lymphoblast localization in the small intestine. Immunology. 1983 Aug;49(4):641–648. [PMC free article] [PubMed] [Google Scholar]
- Ottaway C. A., Manson-Smith D. F., Bruce R. G., Parrott D. M. Regional blood flow and the localization of lymphoblasts in the small intestine of the mouse. II. The effects of a primary enteric infection with Trichinella spiralis. Immunology. 1980 Dec;41(4):963–971. [PMC free article] [PubMed] [Google Scholar]
- Ottaway C. A., Parrott D. M. Regional blood flow and the localisation of lymphoblasts in the small intestine of the mouse: effect of an elemental diet. Gut. 1981 May;22(5):376–382. doi: 10.1136/gut.22.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991 Apr;65(4):2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Regulation of lymphocyte traffic to mucosa-associated lymphatic tissues. Gastroenterol Clin North Am. 1991 Sep;20(3):495–510. [PubMed] [Google Scholar]
- Shou J., Lappin J., Daly J. M. Impairment of pulmonary macrophage function with total parenteral nutrition. Ann Surg. 1994 Mar;219(3):291–297. doi: 10.1097/00000658-199403000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitren H. S., Heller P. A., Bailey L. B., Cerda J. J. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983 Nov-Dec;7(6):582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Miura S., Tashiro H., Serizawa H., Hamada Y., Yoshioka M., Tsuchiya M. Morphological alteration of gut-associated lymphoid tissue after long-term total parenteral nutrition in rats. Cell Tissue Res. 1991 Oct;266(1):29–36. doi: 10.1007/BF00678708. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Mechanisms of immune regulation at mucosal surfaces. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S784–S792. doi: 10.1093/clinids/5.supplement_4.s784. [DOI] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



