Abstract
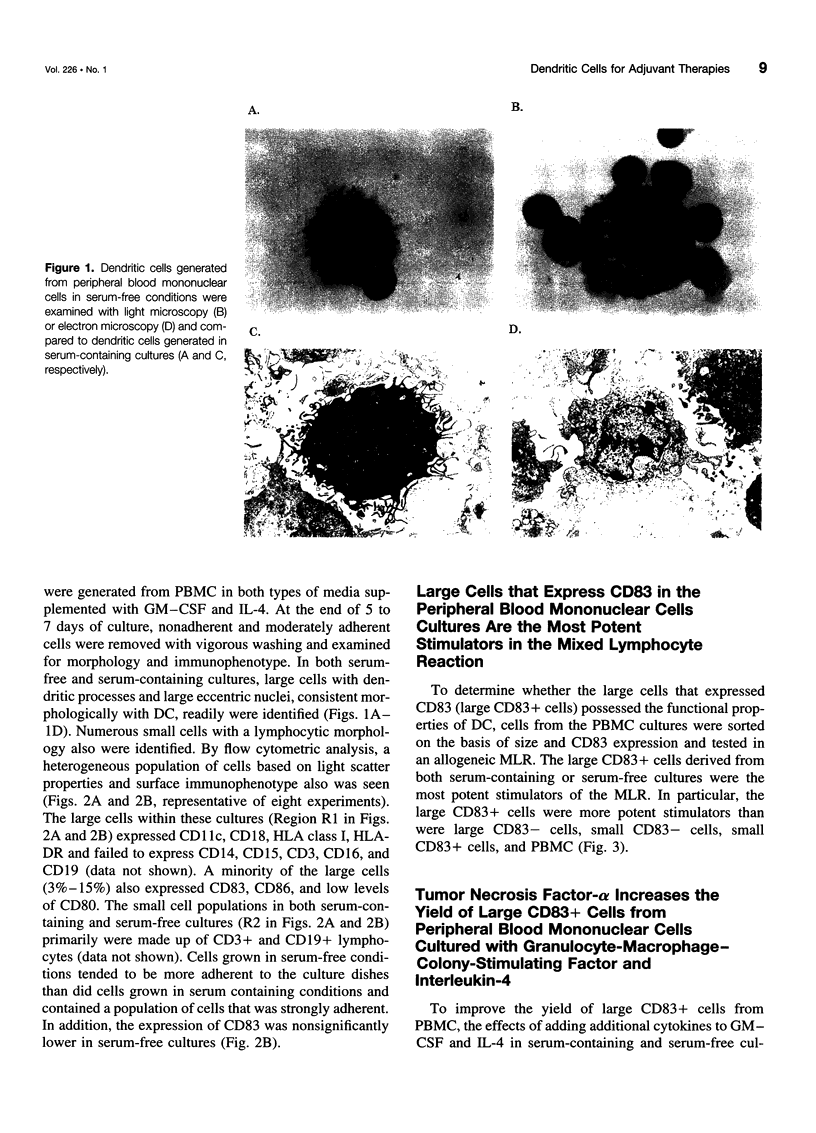
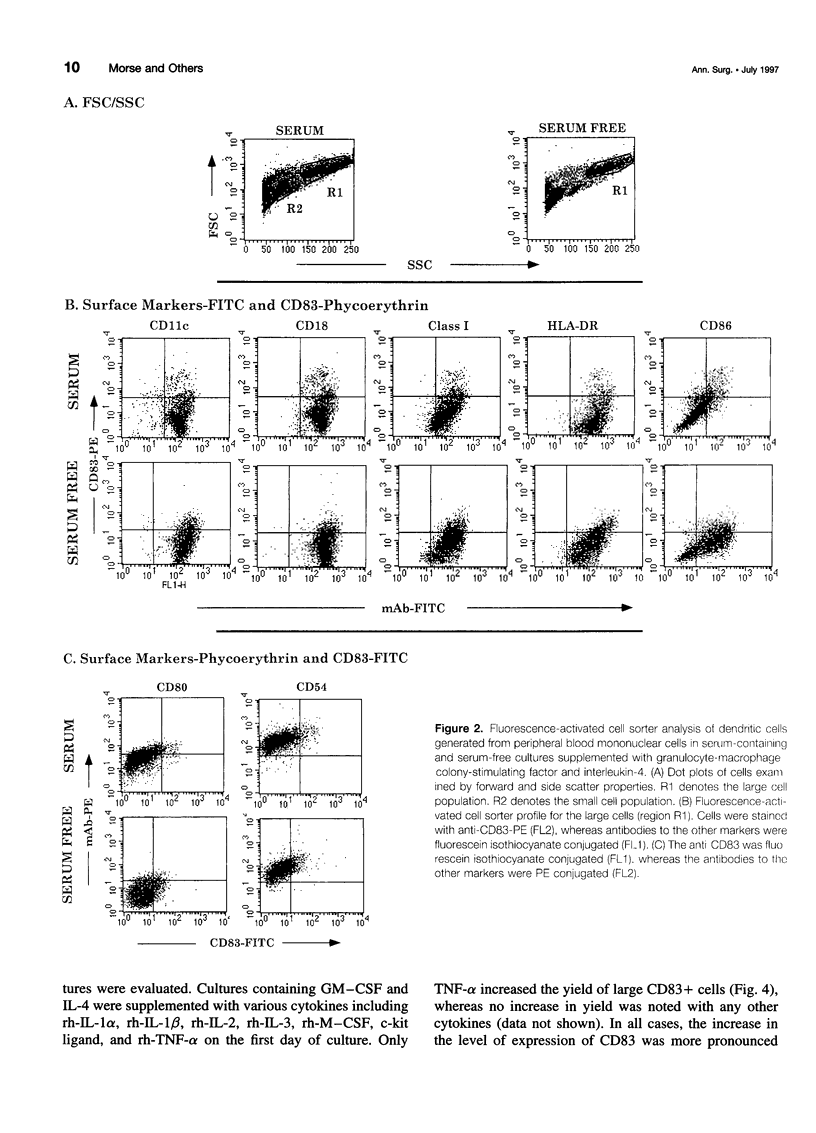
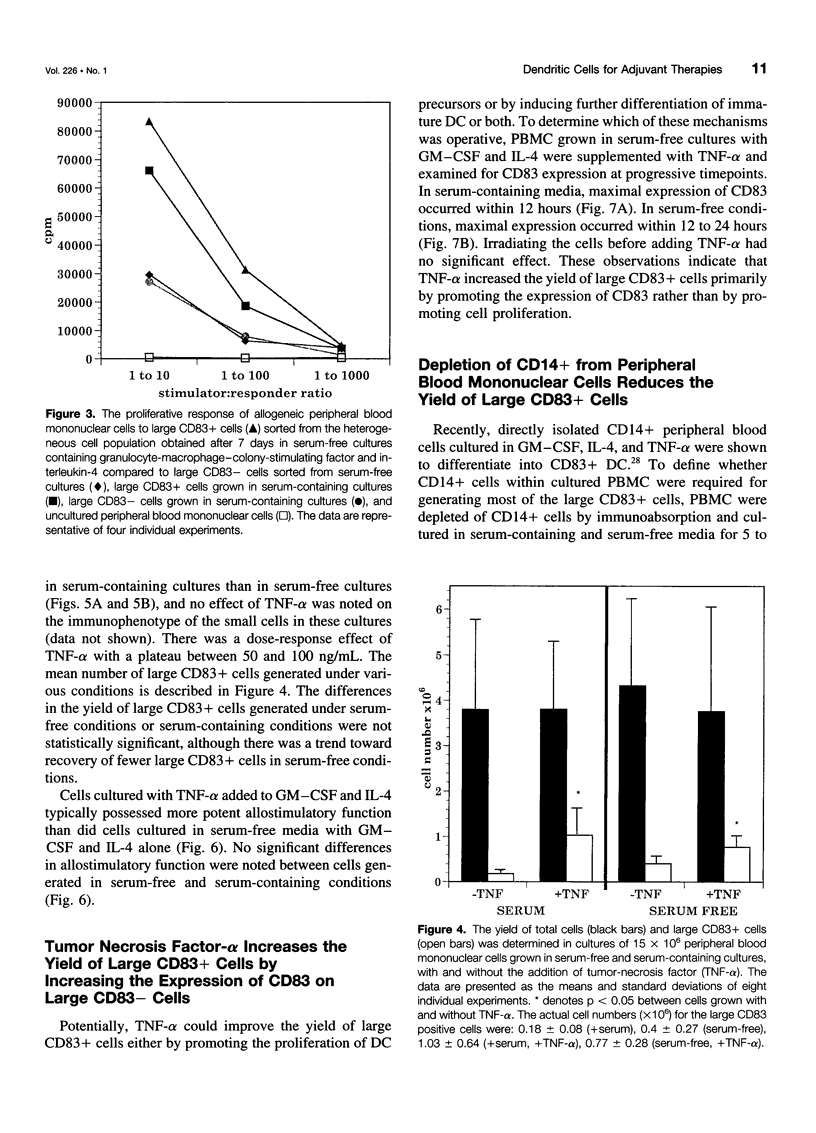
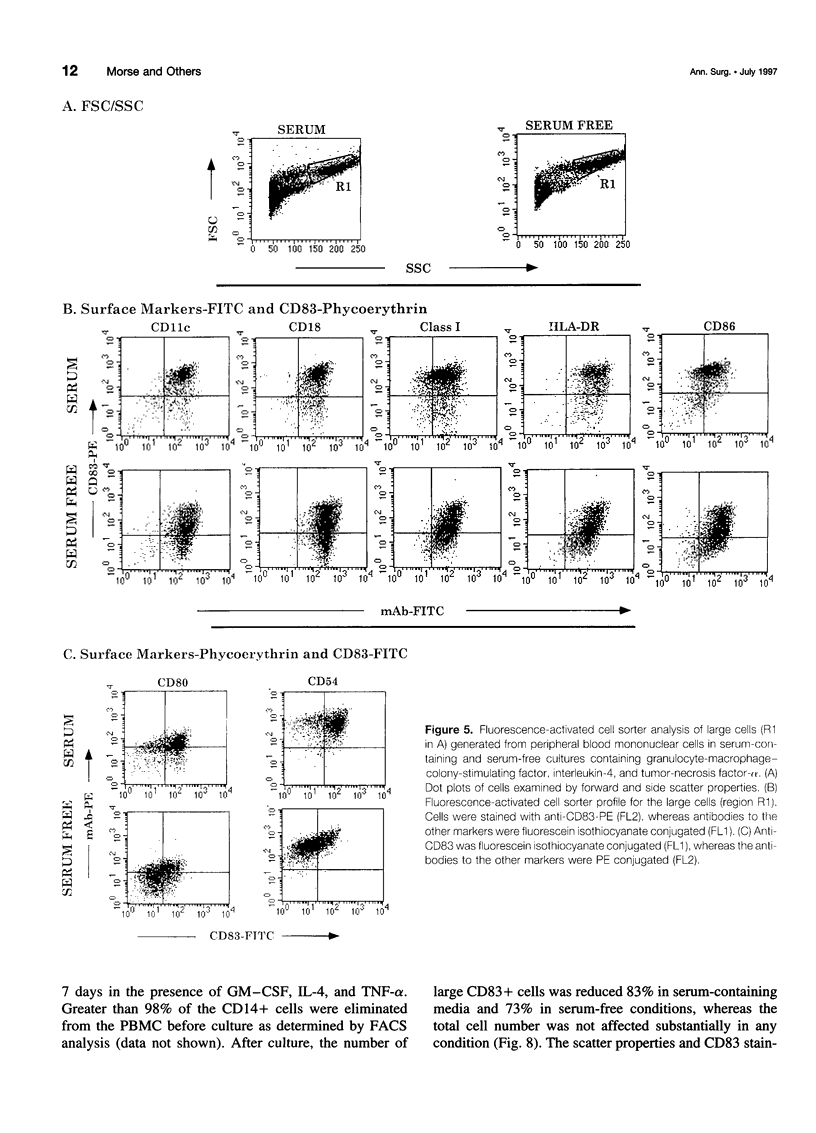
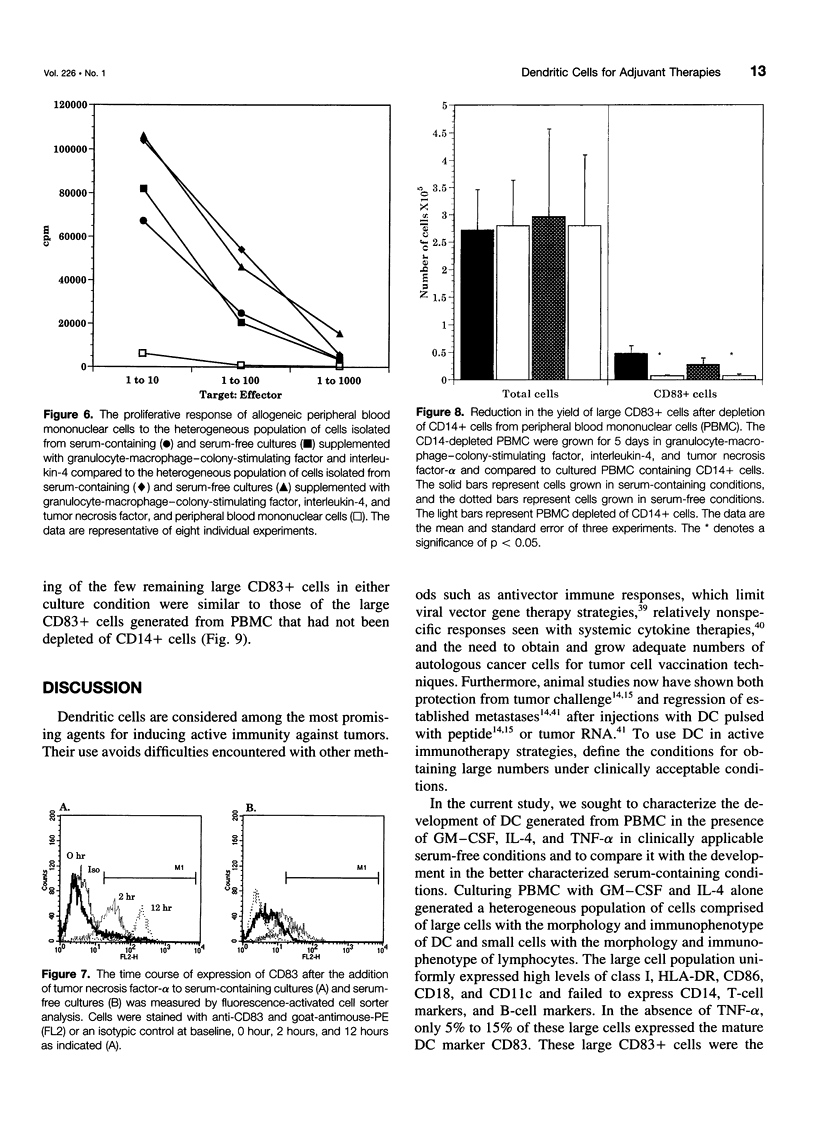
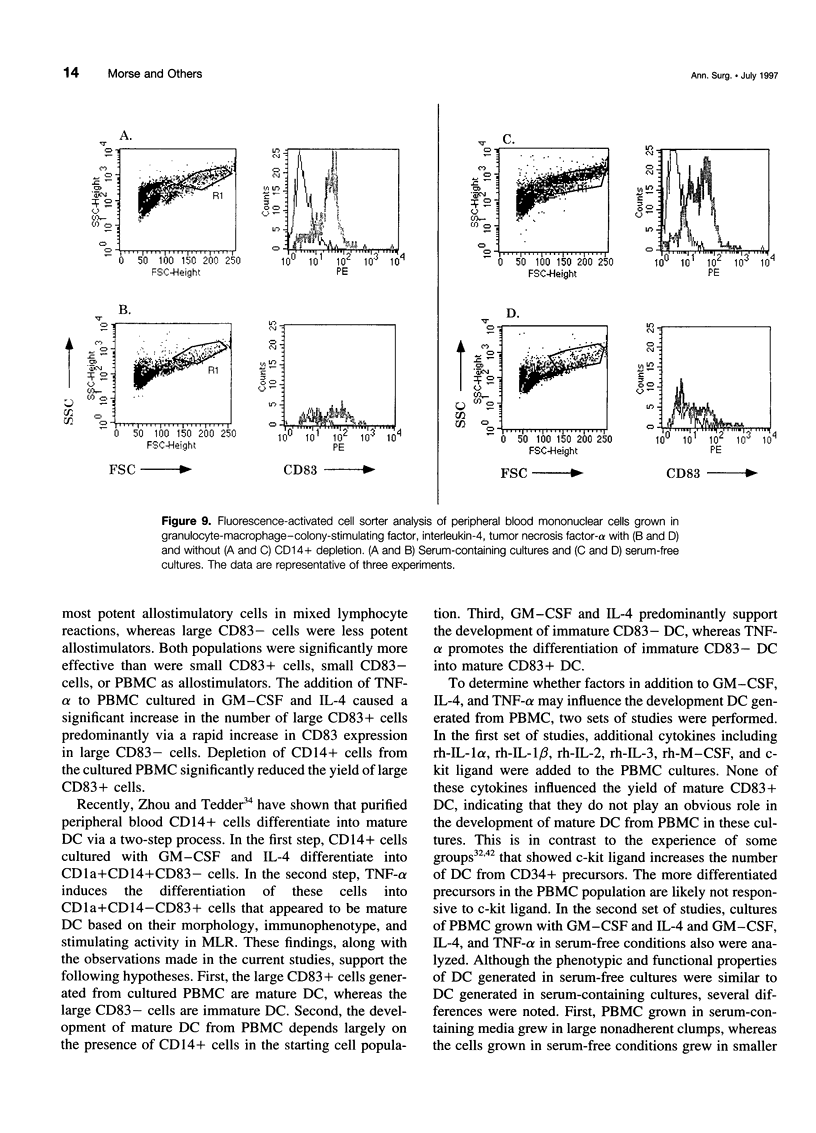
OBJECTIVE: The purpose of the study was to characterize the requirements in terms of precursors, developmental pathways, and media for the generation of large numbers of mature dendritic cells (DC) under conditions acceptable for use in adjuvant, active immunotherapy strategies for surgically treated malignancies. SUMMARY BACKGROUND DATA: Although limited previously by the small numbers accessible, DC-based immunotherapies for malignancy have become more realistic with the development of methods for efficiently generating larger numbers of DC from peripheral blood mononuclear cells (PBMC) in vitro, but these methods rely on clinically unacceptable culture conditions (such as inclusion of fetal bovine serum), necessitating the development of methods for generating functionally equivalent DC in serum-free conditions. METHODS: Plastic-adherent PBMC (from healthy donors and patients with cancer) were incubated for 7 days with granulocyte-macrophage-colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) with and without tumor necrosis factor-alpha (TNF-alpha) in fetal bovine serum-containing and serum-free media and were analyzed by Wright's stain for morphology, flow cytometry for phenotype, and mixed lymphocyte reaction for allostimulatory function. RESULTS: Growth in either serum-containing or serum-free media supplemented with GM-CSF and IL-4 yielded a similarly heterogeneous population of cells, 6% to 10% of which had the morphology (large cells with thin projections), immunophenotype (including CD83+), and function of mature DC. Tumor necrosis factor-alpha significantly augmented the number of these mature DC, whereas preculture depletion of CD14+ PBMC virtually eliminated them. CONCLUSIONS: Generation of mature DC in the authors' serum-free clinically applicable conditions is similar to serum-containing conditions and requires CD14+ precursors, differentiation through a CD14-CD83- immature stage under the influence of GM-CSF and IL-4, and maturation into a CD83+ DC under the influence of TNF-alpha.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boczkowski D., Nair S. K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996 Aug 1;184(2):465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992 Nov 19;360(6401):258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996 Aug 1;184(2):695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celluzzi C. M., Mayordomo J. I., Storkus W. J., Lotze M. T., Falo L. D., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996 Jan 1;183(1):283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. J., Cohen P. A., Rosenberg S. A., Katz S. I., Mulé J. J. Murine epidermal Langerhans cells and splenic dendritic cells present tumor-associated antigens to primed T cells. Eur J Immunol. 1994 Feb;24(2):315–319. doi: 10.1002/eji.1830240206. [DOI] [PubMed] [Google Scholar]
- Egner W., Andreesen R., Hart D. N. Allostimulatory cells in fresh human blood: heterogeneity in antigen-presenting cell populations. Transplantation. 1993 Oct;56(4):945–950. doi: 10.1097/00007890-199310000-00032. [DOI] [PubMed] [Google Scholar]
- Flamand V., Sornasse T., Thielemans K., Demanet C., Bakkus M., Bazin H., Tielemans F., Leo O., Urbain J., Moser M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994 Mar;24(3):605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- Hsu F. J., Benike C., Fagnoni F., Liles T. M., Czerwinski D., Taidi B., Engleman E. G., Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996 Jan;2(1):52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalser M. H., Ellenberg S. S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985 Aug;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowicz S., Engleman E. G. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990 Mar;85(3):955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayordomo J. I., Zorina T., Storkus W. J., Zitvogel L., Celluzzi C., Falo L. D., Melief C. J., Ildstad S. T., Kast W. M., Deleo A. B. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995 Dec;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- Moertel C. G., Fleming T. R., Macdonald J. S., Haller D. G., Laurie J. A., Goodman P. J., Ungerleider J. S., Emerson W. A., Tormey D. C., Glick J. H. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990 Feb 8;322(6):352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- Nestle F. O., Zheng X. G., Thompson C. B., Turka L. A., Nickoloff B. J. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993 Dec 1;151(11):6535–6545. [PubMed] [Google Scholar]
- Ossevoort M. A., Feltkamp M. C., van Veen K. J., Melief C. J., Kast W. M. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J Immunother Emphasis Tumor Immunol. 1995 Aug;18(2):86–94. doi: 10.1097/00002371-199508000-00002. [DOI] [PubMed] [Google Scholar]
- Porgador A., Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995 Jul 1;182(1):255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P. O., Steinman R. M., Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994 Jul 1;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994 Apr 1;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Belilos E., Diamond B., Carsons S. E. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992 Sep;52(3):274–281. [PubMed] [Google Scholar]
- Santiago-Schwarz F., Divaris N., Kay C., Carsons S. E. Mechanisms of tumor necrosis factor-granulocyte-macrophage colony-stimulating factor-induced dendritic cell development. Blood. 1993 Nov 15;82(10):3019–3028. [PubMed] [Google Scholar]
- Siena S., Di Nicola M., Bregni M., Mortarini R., Anichini A., Lombardi L., Ravagnani F., Parmiani G., Gianni A. M. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995 Dec;23(14):1463–1471. [PubMed] [Google Scholar]
- Sornasse T., Flamand V., De Becker G., Bazin H., Tielemans F., Thielemans K., Urbain J., Leo O., Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992 Jan 1;175(1):15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. Dendritic cells and immune-based therapies. Exp Hematol. 1996 Jul;24(8):859–862. [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Szabolcs P., Moore M. A., Young J. W. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995 Jun 1;154(11):5851–5861. [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Tsang K. Y., Zaremba S., Nieroda C. A., Zhu M. Z., Hamilton J. M., Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995 Jul 5;87(13):982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. A., Egner W., Hart D. N. Isolation and function of human dendritic cells. Int Rev Cytol. 1994;153:41–103. doi: 10.1016/s0074-7696(08)62188-9. [DOI] [PubMed] [Google Scholar]
- Xu H., Friedrichs U., Gieseler R. K., Ruppert J., Ocklind G., Peters J. H. Human blood dendritic cells exhibit a distinct T-cell-stimulating mechanism and differentiation pattern. Scand J Immunol. 1992 Nov;36(5):689–696. doi: 10.1111/j.1365-3083.1992.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992 Jul 15;149(2):735–742. [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1995 Nov 1;86(9):3295–3301. [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995 Apr 15;154(8):3821–3835. [PubMed] [Google Scholar]