Abstract
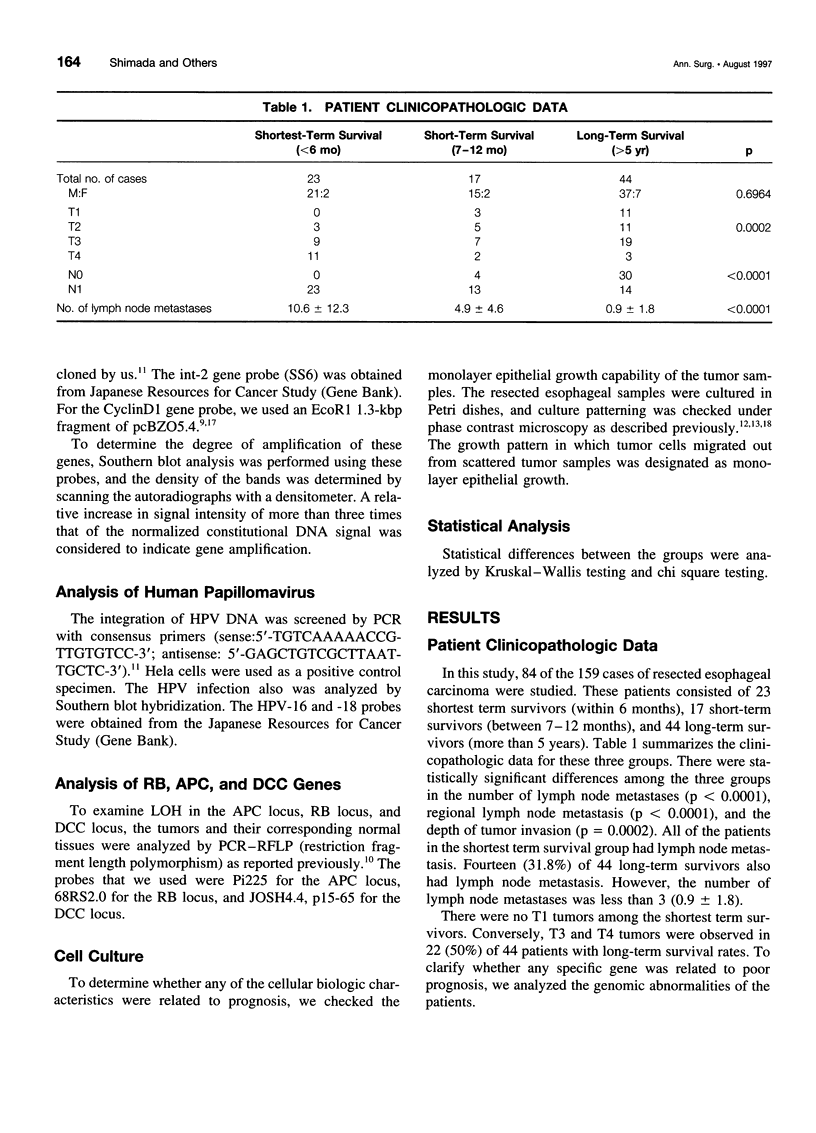
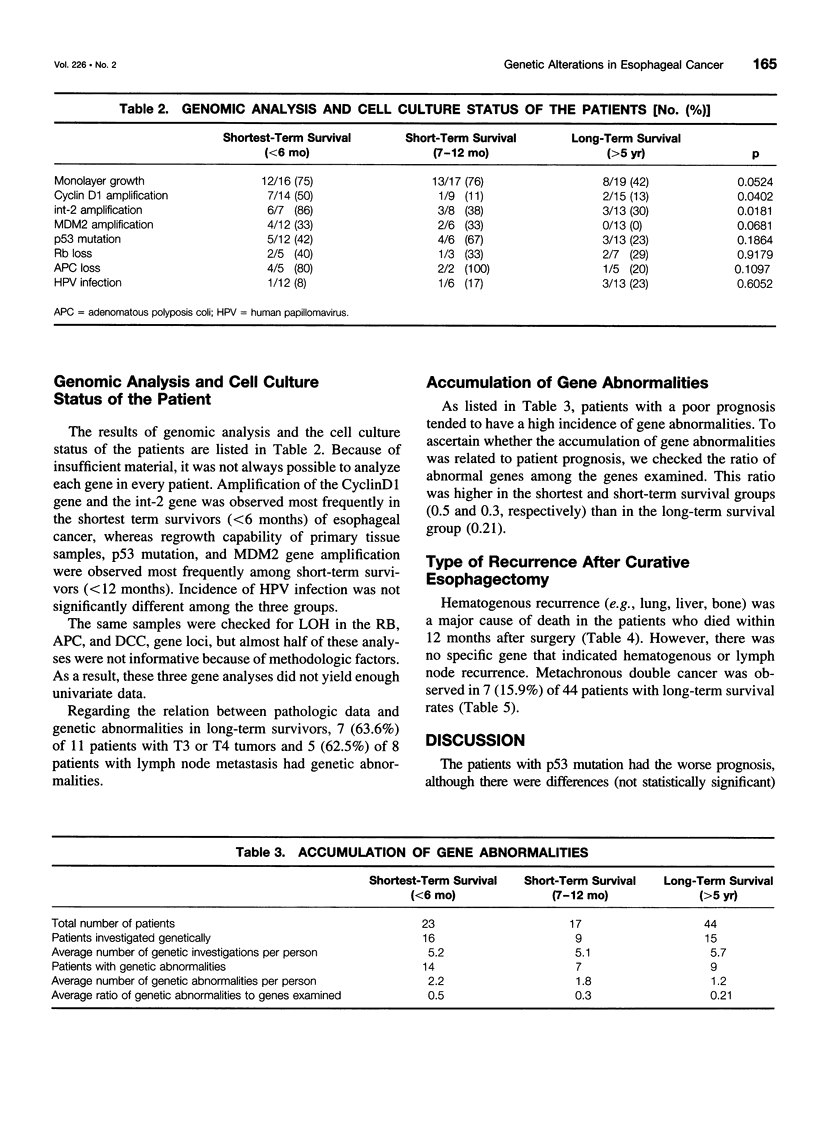
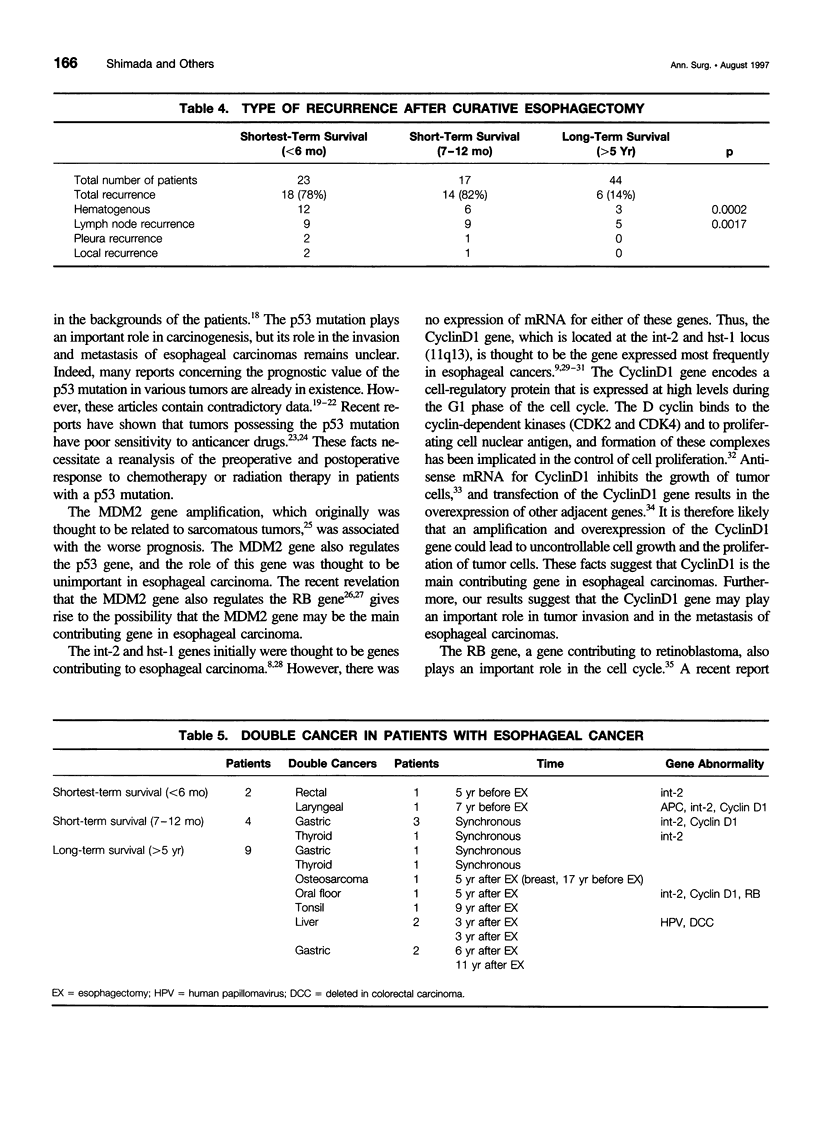
OBJECTIVE: The objective of this study was to ascertain the exact relation between specific oncogenes and long- and short-term survival rates in patients with esophageal cancer. SUMMARY BACKGROUND DATA: Recent developments in molecular biology have shown that several oncogenes and suppressor genes are involved in the development of esophageal cancer. However, the role of these genes still is unknown. METHODS: The clinical outcome of 84 cases of R0-resected esophageal carcinomas (from 1986-1993) and the molecular and biologic characteristics of these tumors were studied. The patients studied were divided into three groups, which were designated as follows: shortest term survivors (up to 6 months), short-term survivors (7-12 months), and long-term survivors (>5 years). These groups included 23, 17, and 44 subjects, respectively. For the genomic analysis, CyclinD1, int-2, murine double minute 2 (MDM2), retinoblastoma, p53, adenomatous polyposis coli (APC), deleted in colorectal carcinoma (DCC), and human papillomavirus were studied in these patients. The regrowth capability of primary cultures and the clinicopathologic characteristics of these patients also were analyzed. RESULTS: The CyclinD1 and int-2 genes, which are located in the 11q13 chromosome, and the MDM2 gene were related to short survival. However, the p53 mutation and human papillomavirus infection were not related to short-term survival. The average ratio of genomic abnormalities to genes examined was higher in the shortest and short-term survival groups than in the long-term survival group. Regrowth capability in primary cultures also was related to short-term survival. Among the long-term survival patients, 7 (16%) of 44 cases suffered further cancer after esophagectomy. CONCLUSIONS: These results suggest that the 11q13 amplicon and MDM2 may play an important role in the progression of esophageal cancer, and an accumulation of genomic abnormalities may result in poor prognosis. Careful follow-up testing for double cancer is needed in long-term survivors of esophageal cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Tsurumaru M., Udagawa H., Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994 Sep;220(3):364–373. doi: 10.1097/00000658-199409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akslen L. A., Varhaug J. E. Oncoproteins and tumor progression in papillary thyroid carcinoma: presence of epidermal growth factor receptor, c-erbB-2 protein, estrogen receptor related protein, p21-ras protein, and proliferation indicators in relation to tumor recurrences and patient survival. Cancer. 1995 Nov 1;76(9):1643–1654. doi: 10.1002/1097-0142(19951101)76:9<1643::aid-cncr2820760922>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bergh J., Norberg T., Sjögren S., Lindgren A., Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med. 1995 Oct;1(10):1029–1034. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- Bland K. I., Konstadoulakis M. M., Vezeridis M. P., Wanebo H. J. Oncogene protein co-expression. Value of Ha-ras, c-myc, c-fos, and p53 as prognostic discriminants for breast carcinoma. Ann Surg. 1995 Jun;221(6):706–720. doi: 10.1097/00000658-199506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. F., Huang Y., Blount P. L., Reid B. J., Raskind W. H., Haggitt R. C., Newkirk C., Resau J. H., Yin J., McDaniel T. Frequent loss of heterozygosity at the retinoblastoma locus in human esophageal cancers. Cancer Res. 1991 Oct 15;51(20):5766–5769. [PubMed] [Google Scholar]
- Gabbert H. E., Müller W., Schneiders A., Meier S., Hommel G. The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995 Sep 1;76(5):720–726. doi: 10.1002/1097-0142(19950901)76:5<720::aid-cncr2820760503>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Harpole D. H., Jr, Herndon J. E., 2nd, Wolfe W. G., Iglehart J. D., Marks J. R. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res. 1995 Jan 1;55(1):51–56. [PubMed] [Google Scholar]
- Iihara K., Shiozaki H., Tahara H., Kobayashi K., Inoue M., Tamura S., Miyata M., Oka H., Doki Y., Mori T. Prognostic significance of transforming growth factor-alpha in human esophageal carcinoma. Implication for the autocrine proliferation. Cancer. 1993 May 15;71(10):2902–2909. doi: 10.1002/1097-0142(19930515)71:10<2902::aid-cncr2820711004>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Imamura M., Shimada Y., Kanda T., Miyahara T., Hashimoto M., Tobe T., Arai T., Hatano Y. Hemodynamic changes after resection of thoracic duct for en bloc resection of esophageal cancer. Surg Today. 1992;22(3):226–232. doi: 10.1007/BF00308827. [DOI] [PubMed] [Google Scholar]
- Itakura Y., Sasano H., Shiga C., Furukawa Y., Shiga K., Mori S., Nagura H. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994 Aug 1;74(3):795–804. doi: 10.1002/1097-0142(19940801)74:3<795::aid-cncr2820740303>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Tomita N., Zhang Y. J., Lu S. H., Weinstein I. B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992 May 15;52(10):2980–2983. [PubMed] [Google Scholar]
- Kadowaki T., Shiozaki H., Inoue M., Tamura S., Oka H., Doki Y., Iihara K., Matsui S., Iwazawa T., Nagafuchi A. E-cadherin and alpha-catenin expression in human esophageal cancer. Cancer Res. 1994 Jan 1;54(1):291–296. [PubMed] [Google Scholar]
- Kanda Y., Nishiyama Y., Shimada Y., Imamura M., Nomura H., Hiai H., Fukumoto M. Analysis of gene amplification and overexpression in human esophageal-carcinoma cell lines. Int J Cancer. 1994 Jul 15;58(2):291–297. doi: 10.1002/ijc.2910580224. [DOI] [PubMed] [Google Scholar]
- Kitadai Y., Ellis L. M., Takahashi Y., Bucana C. D., Anzai H., Tahara E., Fidler I. J. Multiparametric in situ messenger RNA hybridization analysis to detect metastasis-related genes in surgical specimens of human colon carcinomas. Clin Cancer Res. 1995 Oct;1(10):1095–1102. [PubMed] [Google Scholar]
- Kitagawa Y., Ueda M., Ando N., Shinozawa Y., Shimizu N., Abe O. Significance of int-2/hst-1 coamplification as a prognostic factor in patients with esophageal squamous carcinoma. Cancer Res. 1991 Mar 1;51(5):1504–1508. [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Martin K., Trouche D., Hagemeier C., Sørensen T. S., La Thangue N. B., Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995 Jun 22;375(6533):691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- Marx J. How cells cycle toward cancer. Science. 1994 Jan 21;263(5145):319–321. doi: 10.1126/science.8278804. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Kaise T., Ishiguro M., Nakajima T. Better grading systems for evaluating the degree of lymph node invasion in cancer of the thoracic esophagus. Surg Today. 1994;24(6):500–505. doi: 10.1007/BF01884568. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Morita M., Kuwano H., Matsuda H., Moriguchi S., Sugimachi K. Prognostic significance of argyrophilic nucleolar organizer regions in esophageal carcinoma. Cancer Res. 1991 Oct 1;51(19):5339–5341. [PubMed] [Google Scholar]
- Naitoh H., Shibata J., Kawaguchi A., Kodama M., Hattori T. Overexpression and localization of cyclin D1 mRNA and antigen in esophageal cancer. Am J Pathol. 1995 May;146(5):1161–1169. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Zukerberg L., Togawa K., Meltzer S. J., Nishihara T., Rustgi A. K. Human cyclin D1 oncogene and esophageal squamous cell carcinoma. Cancer. 1995 Aug 15;76(4):541–549. doi: 10.1002/1097-0142(19950815)76:4<541::aid-cncr2820760402>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Nakamori S., Yashima K., Murakami Y., Ishikawa O., Ohigashi H., Imaoka S., Yaegashi S., Konishi Y., Sekiya T. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res. 1995 Feb;86(2):174–181. doi: 10.1111/j.1349-7006.1995.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Shimizu N., Abe O. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer. 1989 Jun 1;63(11):2169–2173. doi: 10.1002/1097-0142(19890601)63:11<2169::aid-cncr2820631117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ruol A., Segalin A., Panozzo M., Stephens J. K., Dalla Palma P., Skinner D. B., Peracchia A., Little A. G. Flow cytometric DNA analysis of squamous cell carcinoma of the esophagus. Cancer. 1990 Mar 1;65(5):1185–1188. doi: 10.1002/1097-0142(19900301)65:5<1185::aid-cncr2820650526>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sarbia M., Porschen R., Borchard F., Horstmann O., Willers R., Gabbert H. E. p53 protein expression and prognosis in squamous cell carcinoma of the esophagus. Cancer. 1994 Oct 15;74(8):2218–2223. doi: 10.1002/1097-0142(19941015)74:8<2218::aid-cncr2820740803>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Shibagaki I., Shimada Y., Wagata T., Ikenaga M., Imamura M., Ishizaki K. Allelotype analysis of esophageal squamous cell carcinoma. Cancer Res. 1994 Jun 1;54(11):2996–3000. [PubMed] [Google Scholar]
- Shibagaki I., Tanaka H., Shimada Y., Wagata T., Ikenaga M., Imamura M., Ishizaki K. p53 mutation, murine double minute 2 amplification, and human papillomavirus infection are frequently involved but not associated with each other in esophageal squamous cell carcinoma. Clin Cancer Res. 1995 Jul;1(7):769–773. [PubMed] [Google Scholar]
- Shimada M., Horii A., Sasaki S., Yanagisawa A., Kato Y., Yamashita K., Okagawa K., Yamasaki K., Ishiguro S., Inoue M. Infrequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers of the esophagus and various other tissues. Jpn J Cancer Res. 1995 Jun;86(6):511–515. doi: 10.1111/j.1349-7006.1995.tb02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Imamura M. Prognostic significance of cell culture in carcinoma of the oesophagus. Br J Surg. 1993 May;80(5):605–607. doi: 10.1002/bjs.1800800518. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Kanda Y., Shibagaki I., Kato M., Watanabe G., Tanaka H., Kano M., Imamura M. Prognostic value of monolayer culture patterning in primary cell culture of oesophageal cancer. Br J Surg. 1996 Aug;83(8):1148–1151. doi: 10.1002/bjs.1800830837. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Masayuki I. [Primary cell culture of esophageal cancer as an indicator of biological malignancy]. Hum Cell. 1994 Dec;7(4):193–198. [PubMed] [Google Scholar]
- Tahara E. Genetic alterations in human gastrointestinal cancers. The application to molecular diagnosis. Cancer. 1995 Mar 15;75(6 Suppl):1410–1417. doi: 10.1002/1097-0142(19950315)75:6+<1410::aid-cncr2820751504>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Wagata T., Ishizaki K., Imamura M., Shimada Y., Ikenaga M., Tobe T. Deletion of 17p and amplification of the int-2 gene in esophageal carcinomas. Cancer Res. 1991 Apr 15;51(8):2113–2117. [PubMed] [Google Scholar]
- Wagata T., Shibagaki I., Imamura M., Shimada Y., Toguchida J., Yandell D. W., Ikenaga M., Tobe T., Ishizaki K. Loss of 17p, mutation of the p53 gene, and overexpression of p53 protein in esophageal squamous cell carcinomas. Cancer Res. 1993 Feb 15;53(4):846–850. [PubMed] [Google Scholar]
- Wang D. Y., Xiang Y. Y., Tanaka M., Li X. R., Li J. L., Shen Q., Sugimura H., Kino I. High prevalence of p53 protein overexpression in patients with esophageal cancer in Linxian, China and its relationship to progression and prognosis. Cancer. 1994 Dec 15;74(12):3089–3096. doi: 10.1002/1097-0142(19941215)74:12<3089::aid-cncr2820741205>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Xiao Z. X., Chen J., Levine A. J., Modjtahedi N., Xing J., Sellers W. R., Livingston D. M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995 Jun 22;375(6533):694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- Zhou P., Jiang W., Weghorst C. M., Weinstein I. B. Overexpression of cyclin D1 enhances gene amplification. Cancer Res. 1996 Jan 1;56(1):36–39. [PubMed] [Google Scholar]
- Zhou P., Jiang W., Zhang Y. J., Kahn S. M., Schieren I., Santella R. M., Weinstein I. B. Antisense to cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer cells. Oncogene. 1995 Aug 3;11(3):571–580. [PubMed] [Google Scholar]


