Abstract
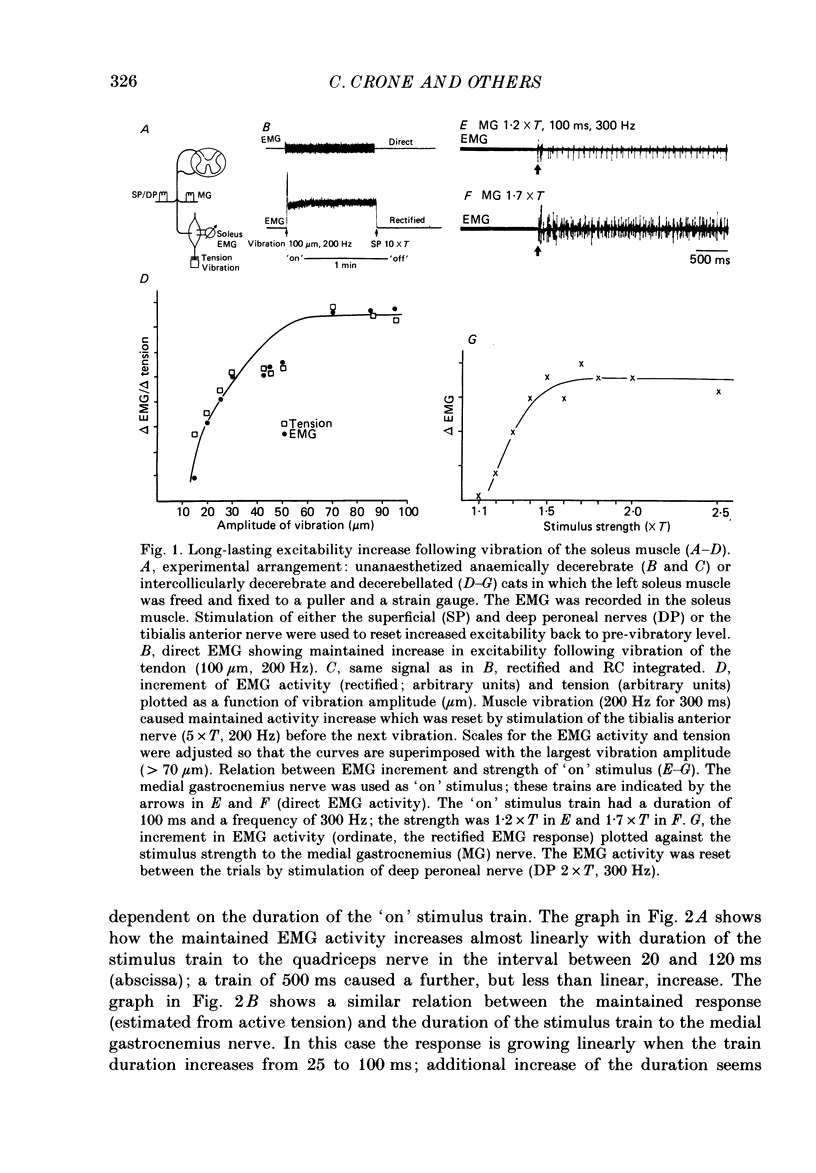
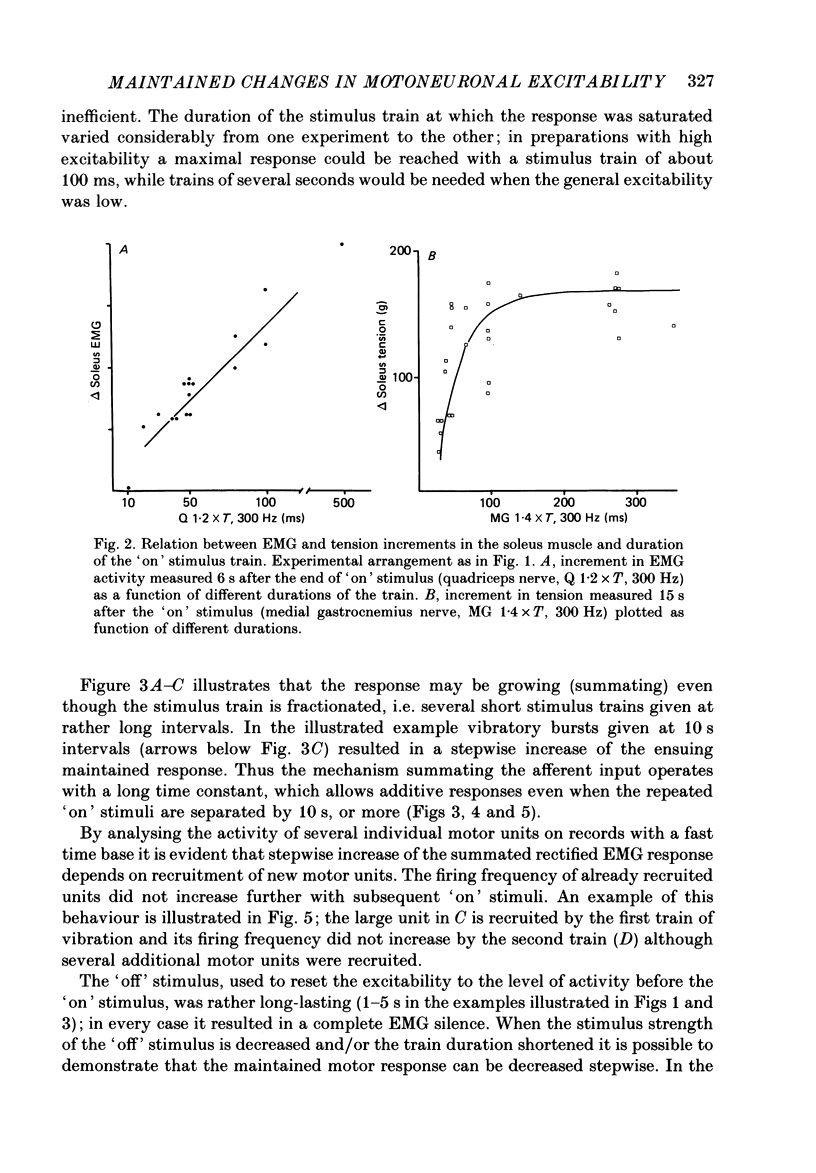
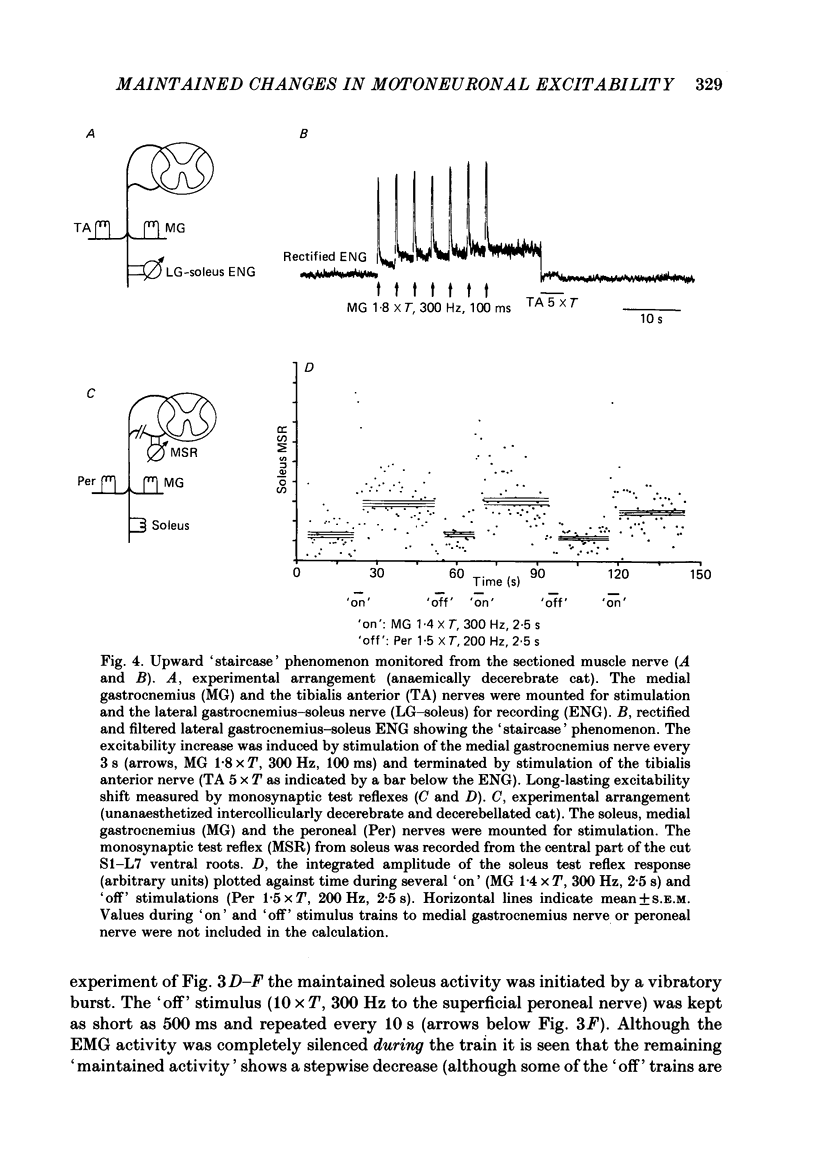
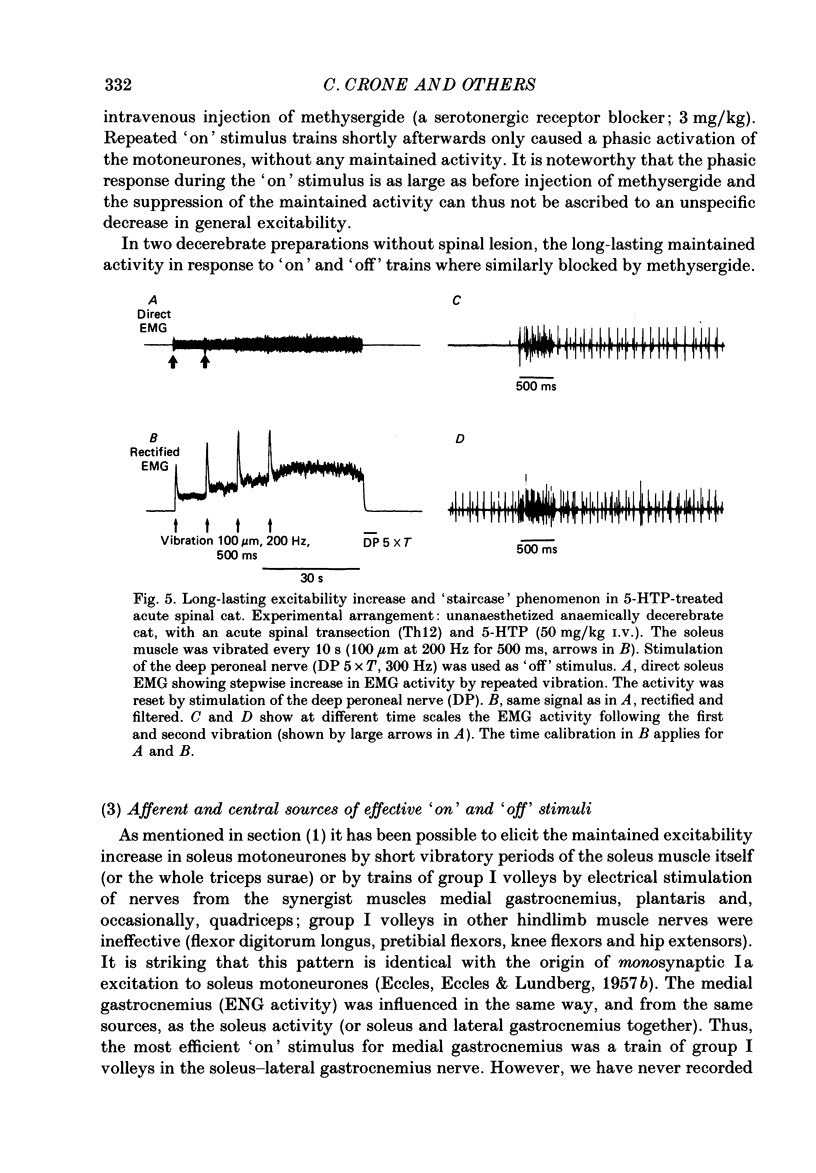
1. During investigation of the tonic stretch reflex in the unanaesthetized decerebrate cat we observed that a short train of impulses in Ia afferents from the soleus muscle (or its synergists) may cause a prolonged activity in the soleus muscle as judged by EMG and tension recordings. This excitability increase, which outlasted the stimulus train, could stay virtually constant during long periods (even minutes), but could be terminated at any time by a train of impulses in, for example, the peroneal nerve. 2. Gradation of the strength of stimulation and the duration of the train of impulses show that the amount of maintained excitability increase depends-within some limits-on the total amount of Ia impulses. 3. In paralysed preparations a short train of impulses in Ia afferents from any part of the triceps surae, caused a maintained increase of the efferent activity in the nerves to triceps surae and a maintained increase of the triceps surae monosynaptic test reflex. These experiments demonstrate the existence of a central mechanism (in the spinal cord and/or the brain stem), which is responsible for the maintained excitability increase seen in motoneurones to the homonymous and synergic muscles. 4. In acute spinal preparations it was not possible to demonstrate any long-lasting excitability increase by a train of Ia impulses. Following intravenous administration of the serotonin precursor 5-hydroxytryptophan, mimicking the tonic activity of these pathways in the decerebrate state, it was again possible to elicit the long-lasting excitability increase by a train of impulses in Ia afferents. A subsequent I.V. injection of methysergide (a serotonin receptor blocker) abolished the long-lasting excitability increase. This set of experiments demonstrates that the basic mechanism responsible for the maintained excitability increase is located at segmental level, and involves serotonergic systems. 5. It was demonstrated that activation of several ipsilateral and crossed reflex pathways by trains of impulses in cutaneous or high-threshold muscle afferents could trigger a maintained excitability increase of those motoneurone pools which were activated by the stimulation. Trains of stimuli to facilitatory regions in the brain stem could also cause a long-lasting excitability increase of motoneurones. Furthermore, activation of all reflex pathways which mediate postsynaptic inhibition to a motor nucleus (including recurrent inhibition via Renshaw cells) could terminate the prolonged excitability increase of that particular motor nucleus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







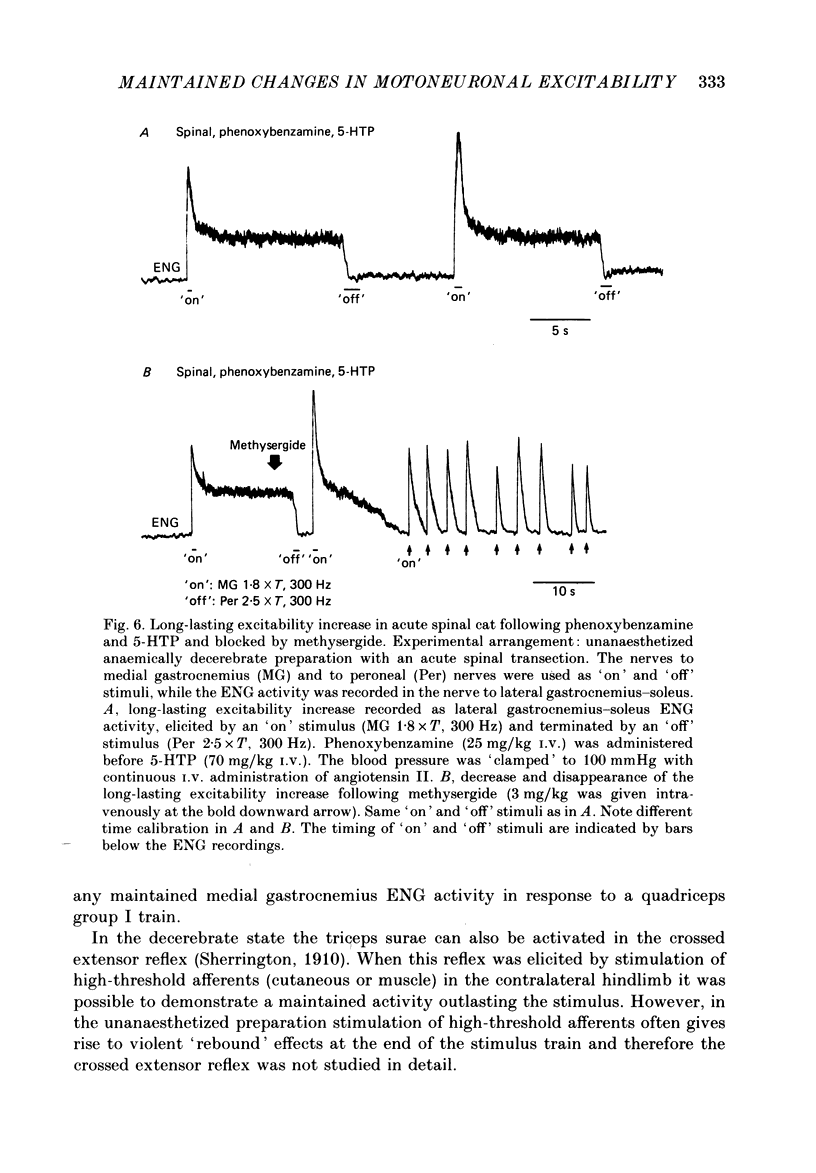

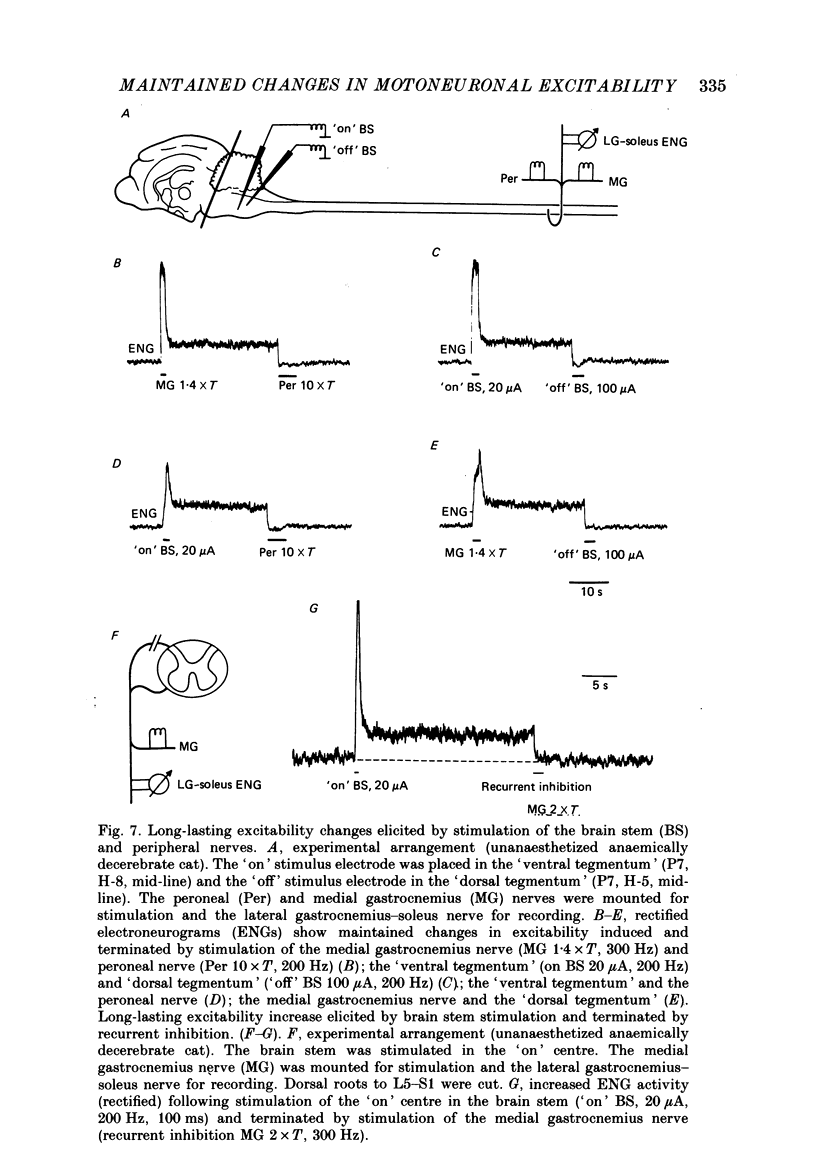








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., JUKES M. G., LUNDBERG A. SPINAL REFLEXES AND MONOAMINE LIBERATION. Nature. 1964 Jun 20;202:1222–1223. doi: 10.1038/2021222a0. [DOI] [PubMed] [Google Scholar]
- ANDEN N. E., JUKES M. G., LUNDBERG A., VYKLICKY L. A NEW SPINAL FLEXOR REFLEX. Nature. 1964 Jun 27;202:1344–1345. doi: 10.1038/2021344b0. [DOI] [PubMed] [Google Scholar]
- Ahlman H., Grillner S., Udo M. The effect of 5-HTP on the static fusimotor activity and the tonic stretch reflex of an extensor muscle. Brain Res. 1971 Apr 2;27(2):393–396. doi: 10.1016/0006-8993(71)90269-1. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. S., Rymer W. Z. Enhancement by serotonin of tonic vibration and stretch reflexes in the decerebrate cat. Exp Brain Res. 1986;62(1):111–122. doi: 10.1007/BF00237407. [DOI] [PubMed] [Google Scholar]
- Conway B. A., Hultborn H., Kiehn O., Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988 Nov;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp Brain Res. 1975;22(2):145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta Physiol Scand. 1968 Jan-Feb;72(1):123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. 3. THE MONOAMINE NERVE TERMINAL. Z Zellforsch Mikrosk Anat. 1965 Feb 9;65:573–596. [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Homma S., Mizote M., Watanabe S. Participation of mono- and polysynaptic transmission during tonic activation of the stretch reflex arcs. Jpn J Physiol. 1975;25(2):135–146. doi: 10.2170/jjphysiol.25.135. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988 Nov;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57(2):422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K. Contribution of polysynaptic pathways to the tonic vibration reflex. Jpn J Physiol. 1972 Aug;22(4):367–377. doi: 10.2170/jjphysiol.22.367. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28(2):161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Mori S., Kawahara K., Sakamoto T., Aoki M., Tomiyama T. Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of brain stem. J Neurophysiol. 1982 Sep;48(3):737–748. doi: 10.1152/jn.1982.48.3.737. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W., Zengel J. E., Lofton S. A., Fleshman J. W. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1982 Nov;48(5):1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Decerebrate Rigidity, and Reflex Coordination of Movements. J Physiol. 1898 Feb 17;22(4):319–332. doi: 10.1113/jphysiol.1898.sp000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]


