Abstract
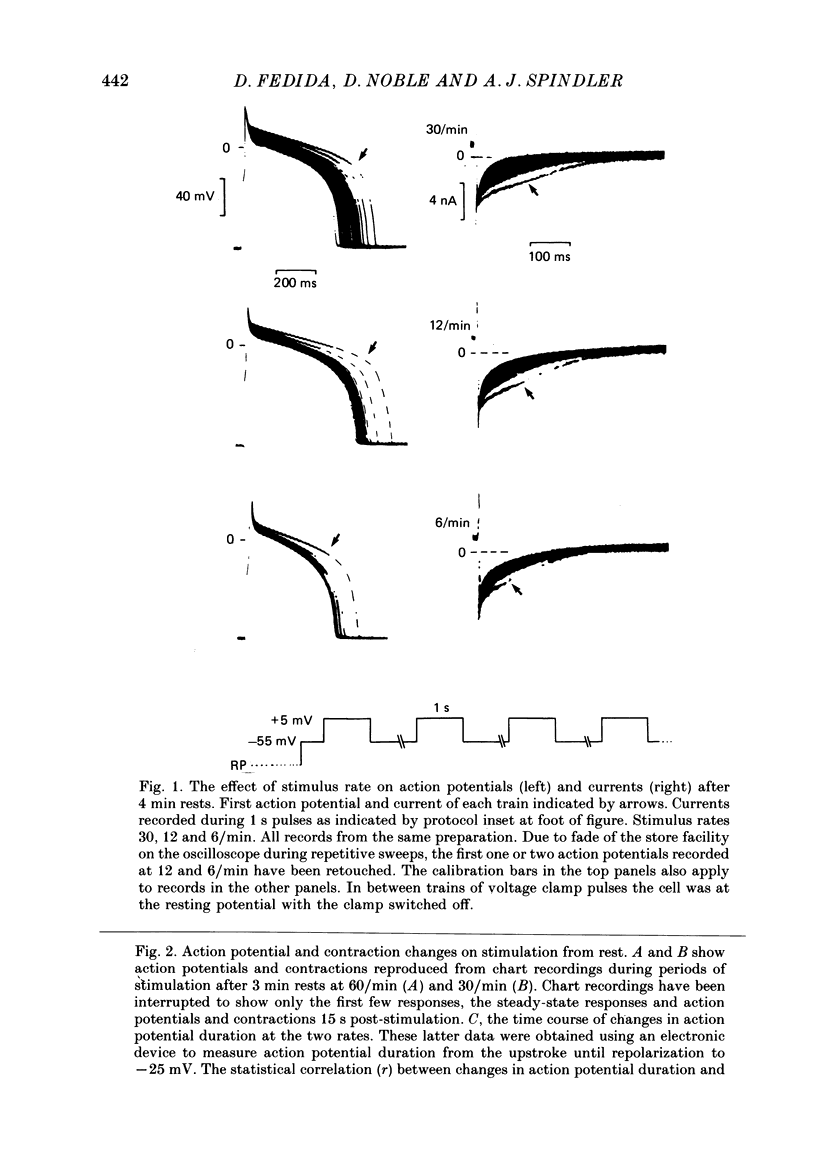
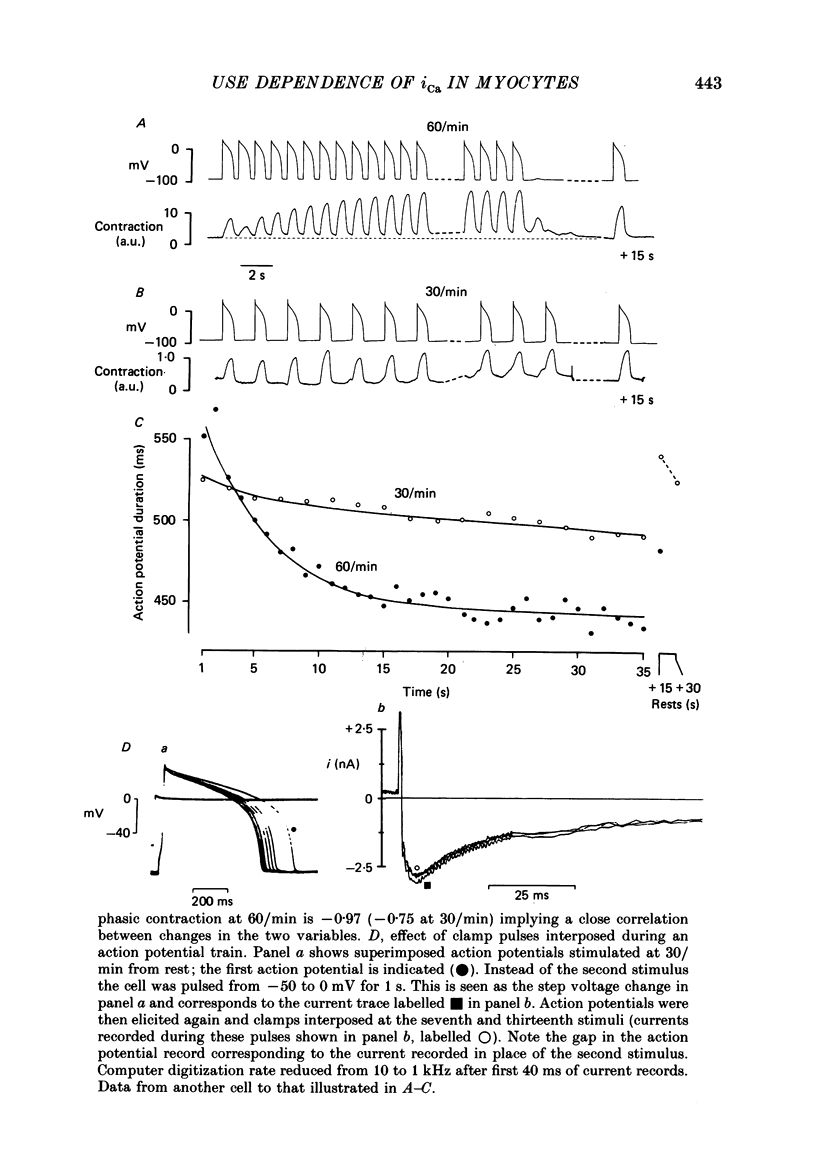
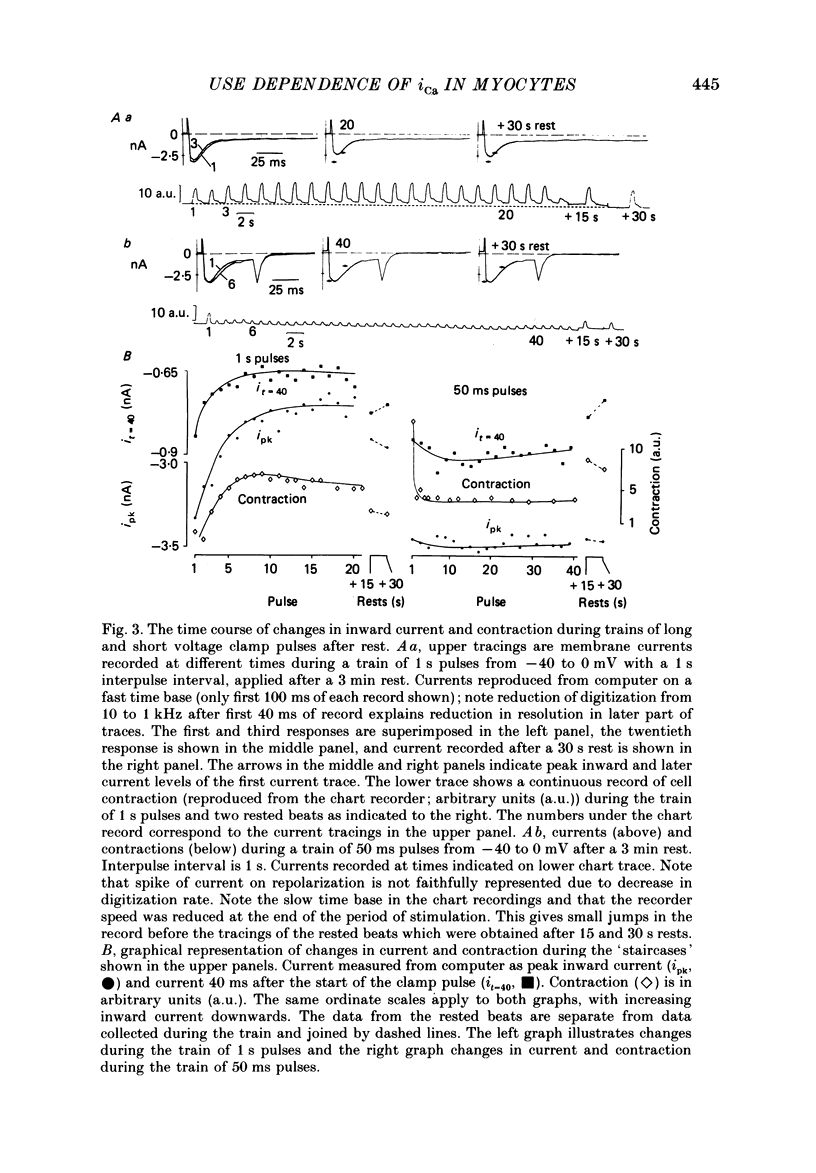
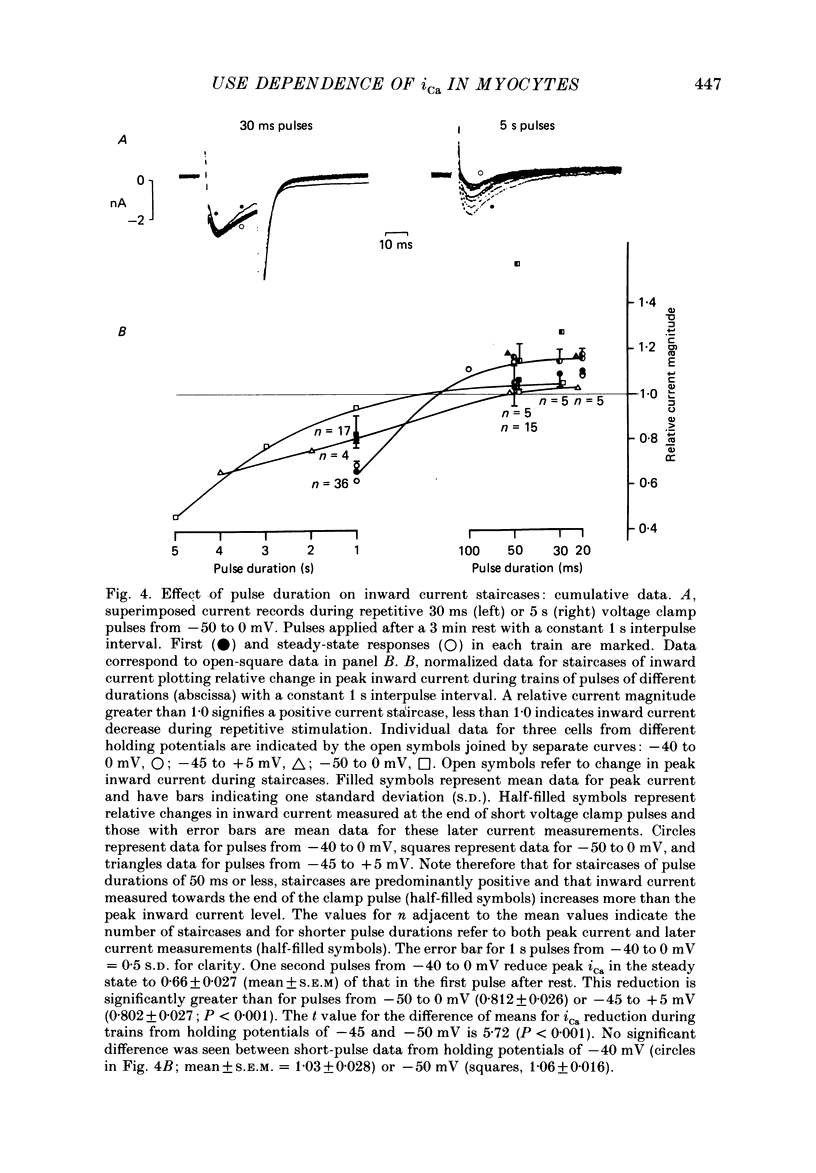
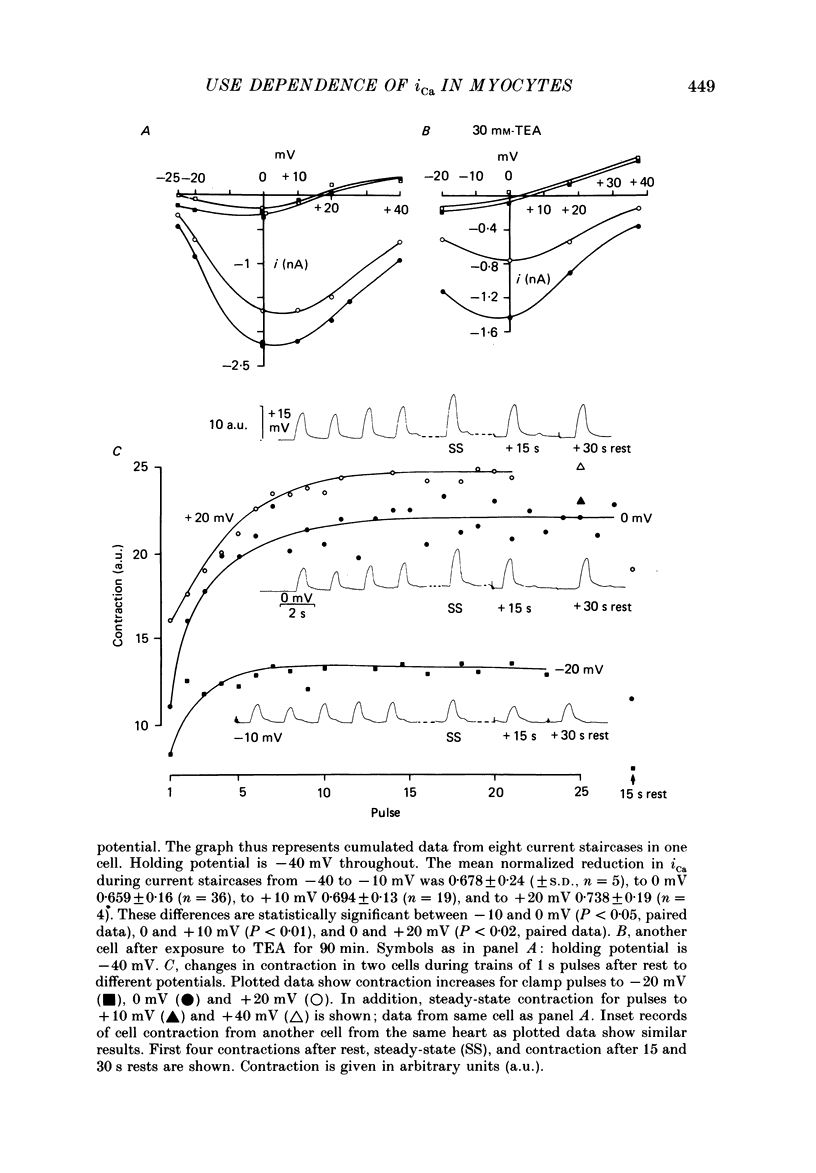
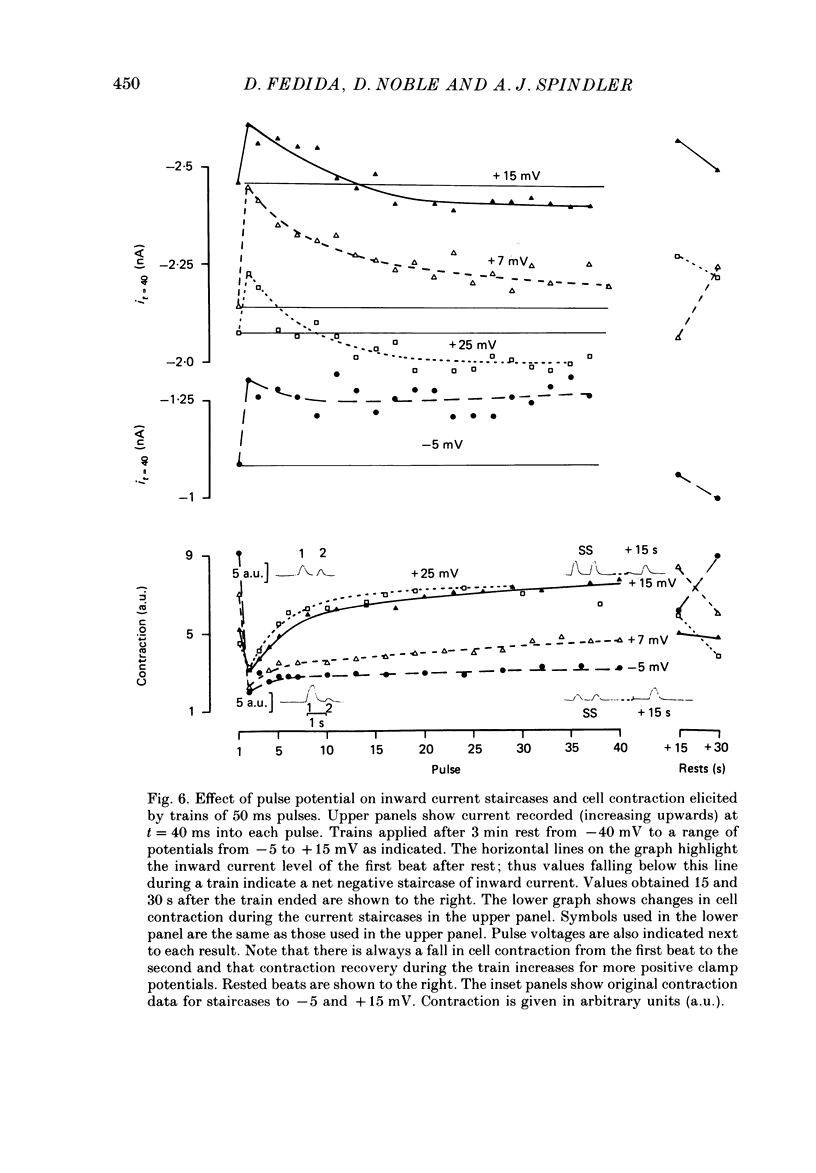
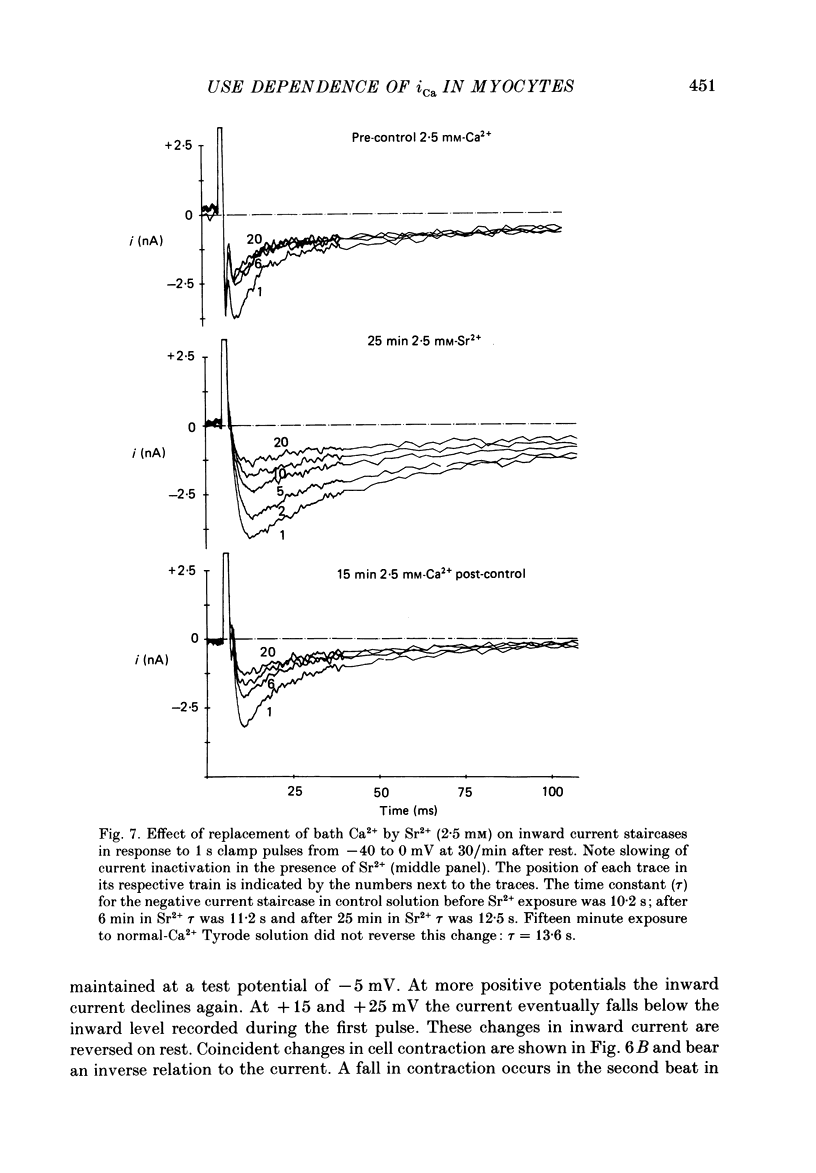
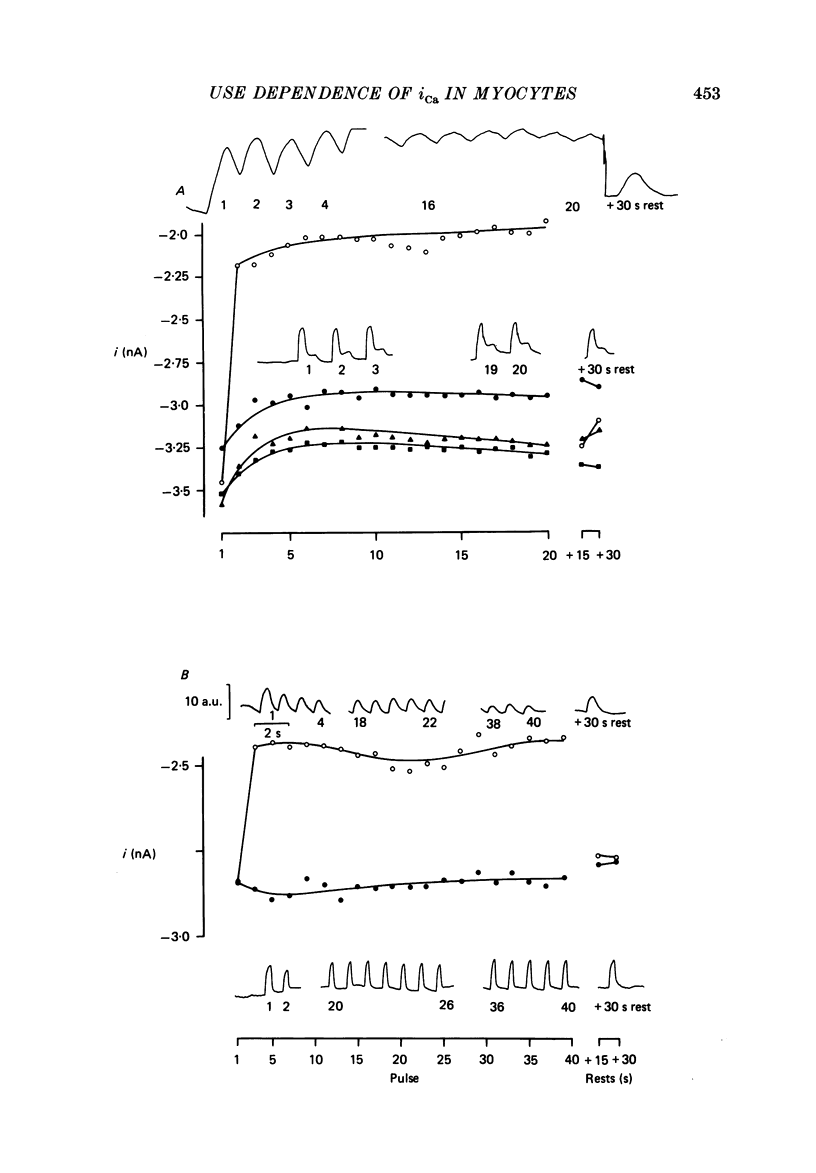
1. Action potentials, calcium currents (iCa) and cell contraction have been recorded from single guinea-pig myocytes during periods of stimulation from rest. Voltage clamp was carried out using a single microelectrode. Cell contraction was measured optically. All experiments were performed at 18-22 degrees C. 2. An inverse relationship was observed between cell contraction and action potential duration or iCa. Mixed trains of action potentials and voltage clamp pulses preserved this relationship. Long voltage clamp pulses induced negative 'staircases' of iCa and positive 'staircases' of cell contraction. A facilitation of iCa was observed during repetitive stimulation with clamp pulses of 100 ms duration or less and was accompanied by a decrease in cell contraction. 3. The voltage dependence of inward current staircases was found to depend on Ca2+ entry rather than membrane voltage for long voltage clamp pulses and was not affected by 30 mM-TEA or 50 microM-TTX. Current reduction was greatest at 0 mV (P less than 0.05) when iCa was largest. Changes in cell contraction during pulse trains showed a similar voltage dependence. The time constant of current staircases was only mildly voltage dependent. 4. Interference with normal cellular mechanisms for Ca2+ uptake and release by strontium, 1-5 mM-caffeine and 1 microM-ryanodine increased current staircases and could abolish iCa facilitation with short clamp pulses. 5. Variations in the level of Ca2+-dependent inactivation of iCa can explain many features of the changes in iCa during stimulation after rest. Long clamp pulses (or action potentials) may increase cell Ca2+ loading and inhibit iCa. Short clamp pulses reduce available Ca2+ for cell contraction and this may reflect a lowered myoplasmic Ca2+ level which allows facilitation of iCa.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Jewell B. R., Wood E. H. Studies of the contractility of mammalian myocardium at low rates of stimulation. J Physiol. 1976 Jan;254(1):1–17. doi: 10.1113/jphysiol.1976.sp011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G. On the relationship between action potential duration and tension in cat papillary muscle. Cardiovasc Res. 1977 May;11(3):210–218. doi: 10.1093/cvr/11.3.210. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Boyett M. R. A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac Purkinje fibres. J Physiol. 1981;319:1–22. doi: 10.1113/jphysiol.1981.sp013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Fedida D. The effect of heart rate on the membrane currents of isolated sheep Purkinje fibres. J Physiol. 1988 May;399:467–491. doi: 10.1113/jphysiol.1988.sp017092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravený P., Sumbera J. Electromechanical correlations in the mammalian heart muscle. Pflugers Arch. 1970;319(1):36–48. doi: 10.1007/BF00586426. [DOI] [PubMed] [Google Scholar]
- Cavalié A., McDonald T. F., Pelzer D., Trautwein W. Temperature-induced transitory and steady-state changes in the calcium current of guinea pig ventricular myocytes. Pflugers Arch. 1985 Oct;405(3):294–296. doi: 10.1007/BF00582574. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- De Hemptinne A., Weyne J. The influence of strontium on the electric activity of cat papillary muscles and Purkinje fibers of sheep hearts. Arch Int Pharmacodyn Ther. 1967 Feb;165(2):494–503. [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Rankin A. C., Spindler A. J. The arrhythmogenic transient inward current iTI and related contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1987 Nov;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Hume J. R. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987 Aug;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W., Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci. 1987 Mar 23;230(1259):163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Scheuer T. Slow inactivation of calcium channels in the cardiac Purkinje fiber. J Mol Cell Cardiol. 1982 Oct;14(10):615–618. doi: 10.1016/0022-2828(82)90148-1. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Herdey A., Kübler M. Interchangeability of Ca ions and Sr ions as charge carriers of the slow inward current in mammalian myocardial fibres. Pflugers Arch. 1973 Nov 26;344(2):149–158. doi: 10.1007/BF00586548. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Electrical activity and contraction in cells isolated from rat and guinea-pig ventricular muscle: a comparative study. J Physiol. 1987 Oct;391:527–544. doi: 10.1113/jphysiol.1987.sp016754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Influence of a change in stimulation rate on action potentials, currents and contractions in rat ventricular cells. J Physiol. 1985 Jul;364:113–130. doi: 10.1113/jphysiol.1985.sp015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. Voltage-dependent potentiation of the slow inward current in frog atrium. J Physiol. 1981 Jan;310:77–95. doi: 10.1113/jphysiol.1981.sp013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter M., Stickel F. J. Der Einfluss der Kontraktionsfrequenz auf das Aktionspotential des Meerschweinchem-Papillarmuskels. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(4):342–365. [PubMed] [Google Scholar]
- Reuter H. Time- and voltage-dependent contractile responses in mammalian cardiac muscle. Eur J Cardiol. 1973 Dec;1(2):177–181. [PubMed] [Google Scholar]
- Simurda J., Simurdova M., Braveny P., Sumbera J. Slow inward current and action potentials of papillary muscles under non-steady state conditions. Pflugers Arch. 1976 Apr 6;362(3):209–218. doi: 10.1007/BF00581172. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


