Abstract
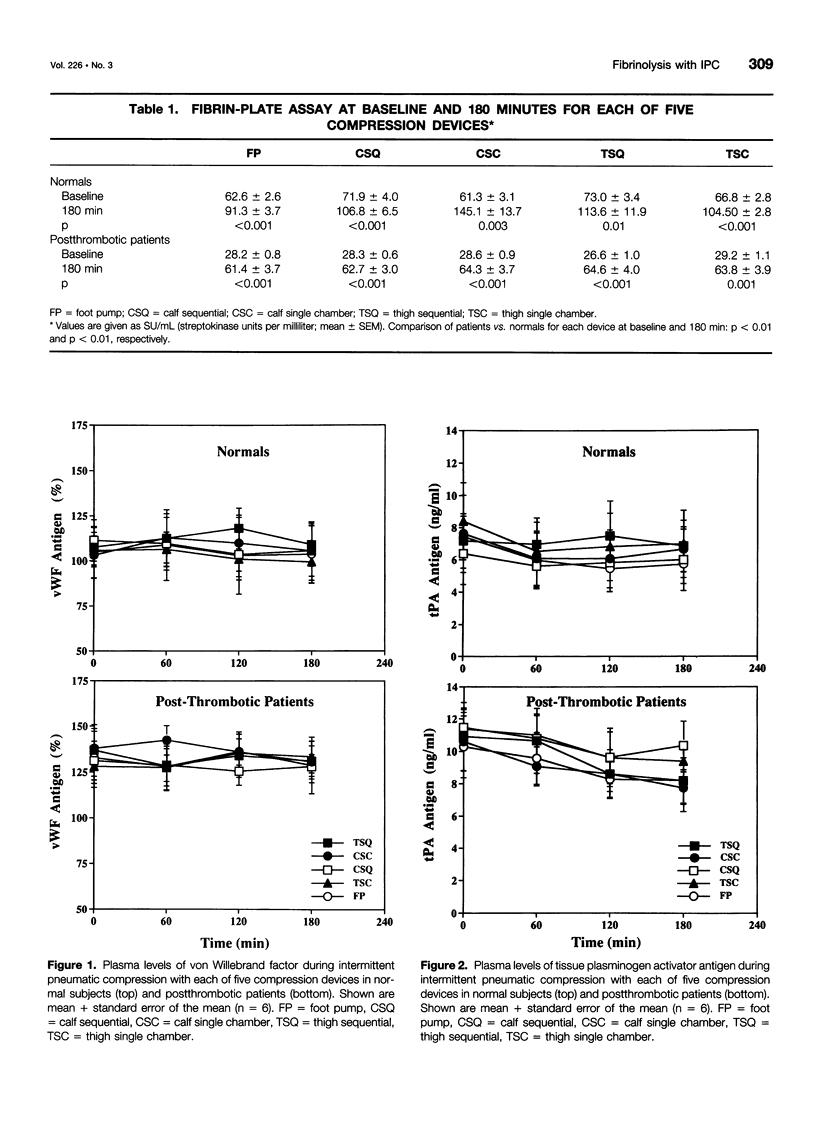
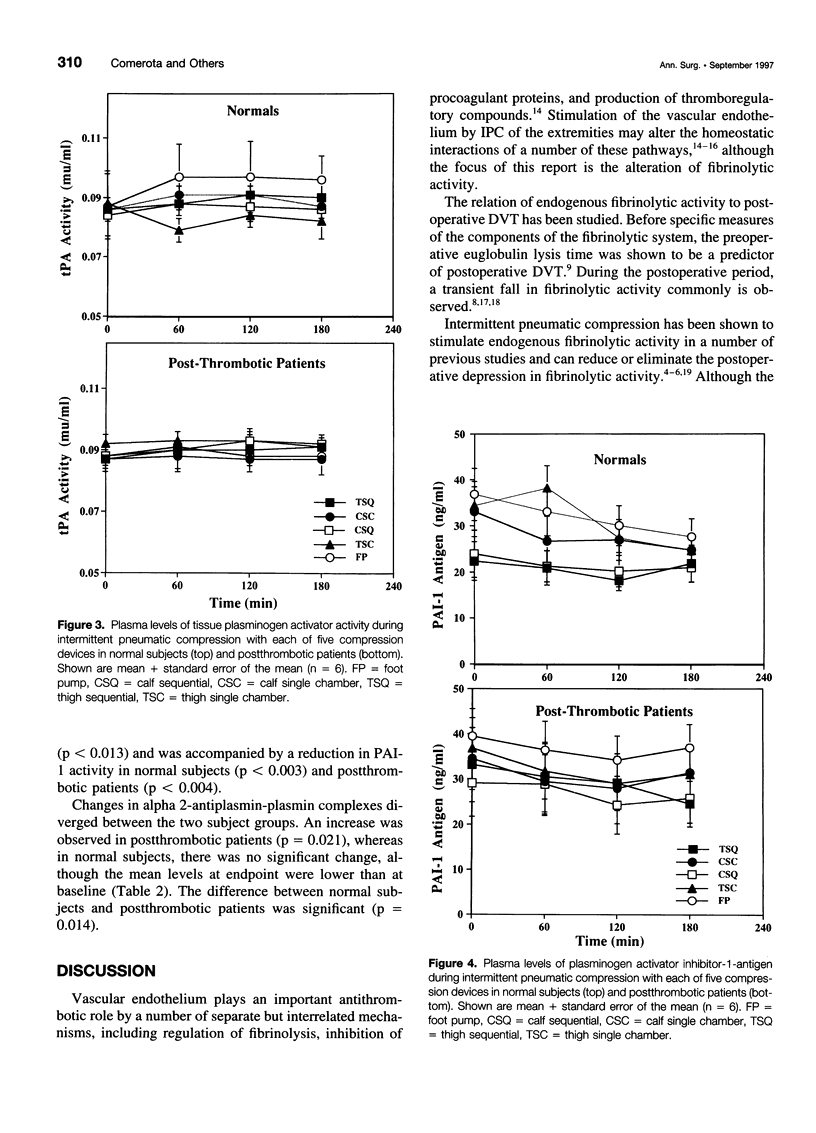
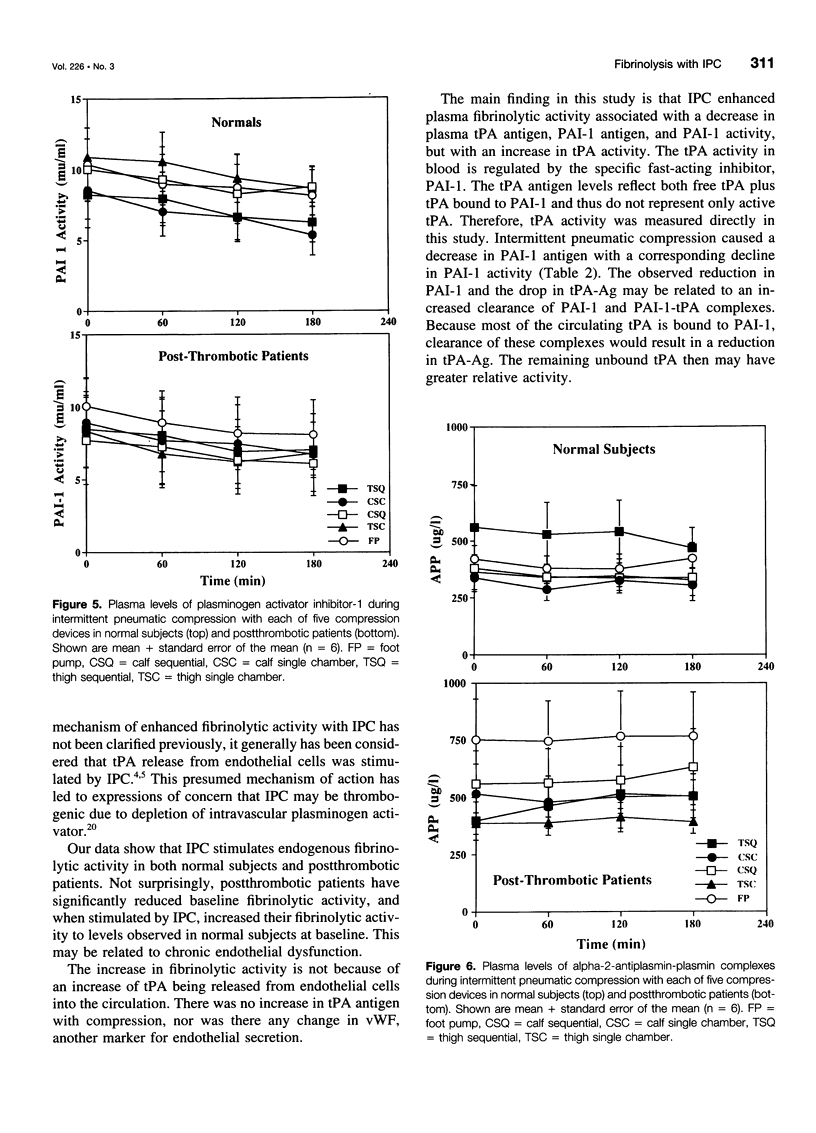
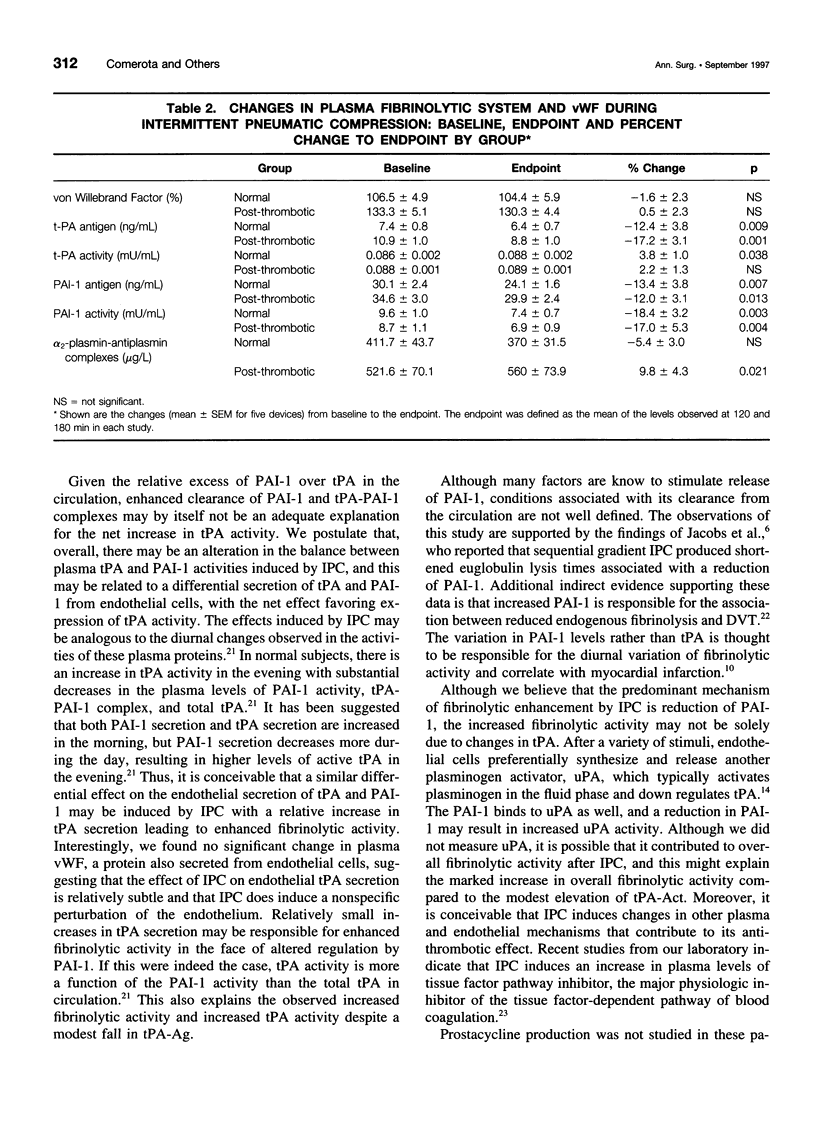
BACKGROUND AND OBJECTIVES: Intermittent pneumatic compression (IPC) is an effective form of deep vein thrombosis prophylaxis for general surgery patients. The antithrombotic effect of IPC is thought to be the result of increased venous velocity and stimulation of endogenous fibrinolysis. However, the mechanism of enhanced fibrinolytic activity and the relative effects on normal and postthrombotic veins have not been defined. The purposes of this study are 1) to quantify changes in fibrinolytic activity with IPC; 2) to study the mechanism of fibrinolytic enhancement with IPC; and 3) to evaluate whether postthrombotic patients have the same capacity for fibrinolytic enhancement with IPC as do normal subjects. METHODS: Twelve volunteers (6 normal and 6 postthrombotic) had 5 IPC devices applied for 120 minutes in random fashion, 1 per week x 5 weeks. The devices included single-chamber, sequential, foot, calf, and long-leg compression. Subjects had an indwelling antecubital venous cannula placed for blood drawn at baseline, 60, 120, and 180 minutes after IPC devices were applied. Global fibrinolytic activity (euglobulin fraction, fibrin plate assay), tissue plasminogen activator (tPA) antigen (Ag) and activity (Act), plasminogen activator inhibitor-1 (PAI-1) Ag and Act, alpha-2-antiplasmin-plasmin complexes, and von Willebrand factor (vWF) antigen were assayed. RESULTS: A striking elevation in fibrinolytic activity was noted at 180 minutes with all devices in normal subjects and postthrombotic patients (p = 0.01-0.0001); however, baseline and stimulated fibrinolytic activity was attenuated in postthrombotic patients (<0.03). The tPA-Act increased only in normal subjects (3.8 +/- 1.9%) (p = 0.057), despite a decrease in plasma tPA-Ag, which was observed in both normal subjects (-12.4 +/- 3.8%) (p = 0.009) and patients (-17.2 +/- 3.1%) (p = 0.001). PAI-1-Ag decreased in both normal subjects (-13.4 +/- 3.8%) (p = 0.007) and patients (-12.0 +/- 3.1%) (p = 0.013) with a marked reduction in PAI-1-Act in both normal subjects (p = 0.003) and patients (p = 0.004). There were no changes in vWF, and alpha-2-antiplasmin-plasmin complexes increased only in postthrombotic patients (p = 0.021). CONCLUSIONS: Stimulation of endogenous fibrinolytic activity occurs after IPC, both in normal subjects and postthrombotic patients; however, baseline and overall fibrinolytic response in postthrombotic patients is reduced. The mechanism of increased fibrinolytic activity is likely because of a reduction in PAI-1, with a resulting increase of tPA activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angleton P., Chandler W. L., Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation. 1989 Jan;79(1):101–106. doi: 10.1161/01.cir.79.1.101. [DOI] [PubMed] [Google Scholar]
- Chandler W. L., Trimble S. L., Loo S. C., Mornin D. Effect of PAI-1 levels on the molar concentrations of active tissue plasminogen activator (t-PA) and t-PA/PAI-1 complex in plasma. Blood. 1990 Sep 1;76(5):930–937. [PubMed] [Google Scholar]
- Clagett G. P., Anderson F. A., Jr, Heit J., Levine M. N., Wheeler H. B. Prevention of venous thromboembolism. Chest. 1995 Oct;108(4 Suppl):312S–334S. doi: 10.1378/chest.108.4_supplement.312s. [DOI] [PubMed] [Google Scholar]
- Clayton J. K., Anderson J. A., McNicol G. P. Preoperative prediction of postoperative deep vein thrombosis. Br Med J. 1976 Oct 16;2(6041):910–912. doi: 10.1136/bmj.2.6041.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. G., Hagen P. O. The vascular endothelium. A new horizon. Ann Surg. 1993 Nov;218(5):593–609. doi: 10.1097/00000658-199321850-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman J. A., Chalmers T. C., Smith H., Jr, Kuebler R. R. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978 Sep 28;299(13):690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- Gertler J. P., Abbott W. M. Prothrombotic and fibrinolytic function of normal and perturbed endothelium. J Surg Res. 1992 Jan;52(1):89–95. doi: 10.1016/0022-4804(92)90284-7. [DOI] [PubMed] [Google Scholar]
- Guyton D. P., Khayat A., Husni E. A., Schreiber H. Elevated levels of 6-keto-prostaglandin-F1a from a lower extremity during external pneumatic compression. Surg Gynecol Obstet. 1988 Apr;166(4):338–342. [PubMed] [Google Scholar]
- Hechtman H. B., Huval W. V., Mathieson M. A., Stemp L. I., Valeri C. R., Shepro D. Prostaglandin and thromboxane mediation of cardiopulmonary failure. Surg Clin North Am. 1983 Apr;63(2):263–283. doi: 10.1016/s0039-6109(16)42981-6. [DOI] [PubMed] [Google Scholar]
- Jacobs D. G., Piotrowski J. J., Hoppensteadt D. A., Salvator A. E., Fareed J. Hemodynamic and fibrinolytic consequences of intermittent pneumatic compression: preliminary results. J Trauma. 1996 May;40(5):710–717. doi: 10.1097/00005373-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Knight M. T., Dawson R., Melrose D. G. Fibrinolytic response to surgery. Labile and stable patterns and their relevance to post-operative deep venous thrombosis. Lancet. 1977 Aug 20;2(8034):370–373. doi: 10.1016/s0140-6736(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Mansfield A. O. Alteration in fibrinolysis associated with surgery and venous thrombosis. Br J Surg. 1972 Oct;59(10):754–757. doi: 10.1002/bjs.1800591004. [DOI] [PubMed] [Google Scholar]
- Nicoloso G., Hauert J., Kruithof E. K., Van Melle G., Bachmann F. Fibrinolysis in normal subjects--comparison between plasminogen activator inhibitor and other components of the fibrinolytic system. Thromb Haemost. 1988 Apr 8;59(2):299–303. [PubMed] [Google Scholar]
- Prins M. H., Hirsh J. A critical review of the evidence supporting a relationship between impaired fibrinolytic activity and venous thromboembolism. Arch Intern Med. 1991 Sep;151(9):1721–1731. [PubMed] [Google Scholar]
- Salzman E. W., McManama G. P., Shapiro A. H., Robertson L. K., Donovan A. S., Blume H. W., Sweeney J., Kamm R. D., Johnson M. C., Black P. M. Effect of optimization of hemodynamics on fibrinolytic activity and antithrombotic efficacy of external pneumatic calf compression. Ann Surg. 1987 Nov;206(5):636–641. doi: 10.1097/00000658-198711000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summaria L., Caprini J. A., McMillan R., Sandesara J., Axelrod C. A., Mueller M. E., Vagher J. P., Walenga J., Fareed J. Relationship between postsurgical fibrinolytic parameters and deep vein thrombosis in surgical patients treated with compression devices. Am Surg. 1988 Mar;54(3):156–160. [PubMed] [Google Scholar]
- Tarnay T. J., Rohr P. R., Davidson A. G., Stevenson M. M., Byars E. F., Hopkins G. R. Pneumatic calf compression, fibrinolysis, and the prevention of deep venous thrombosis. Surgery. 1980 Oct;88(4):489–496. [PubMed] [Google Scholar]
- Tarnay T. J., Rohr P. R., Davidson A. G., Stevenson M. M., Byars E. F., Hopkins G. R. Pneumatic calf compression, fibrinolysis, and the prevention of deep venous thrombosis. Surgery. 1980 Oct;88(4):489–496. [PubMed] [Google Scholar]
- Wiman B., Hamsten A. Impaired fibrinolysis and risk of thromboembolism. Prog Cardiovasc Dis. 1991 Nov-Dec;34(3):179–192. doi: 10.1016/0033-0620(91)90012-b. [DOI] [PubMed] [Google Scholar]


