Abstract
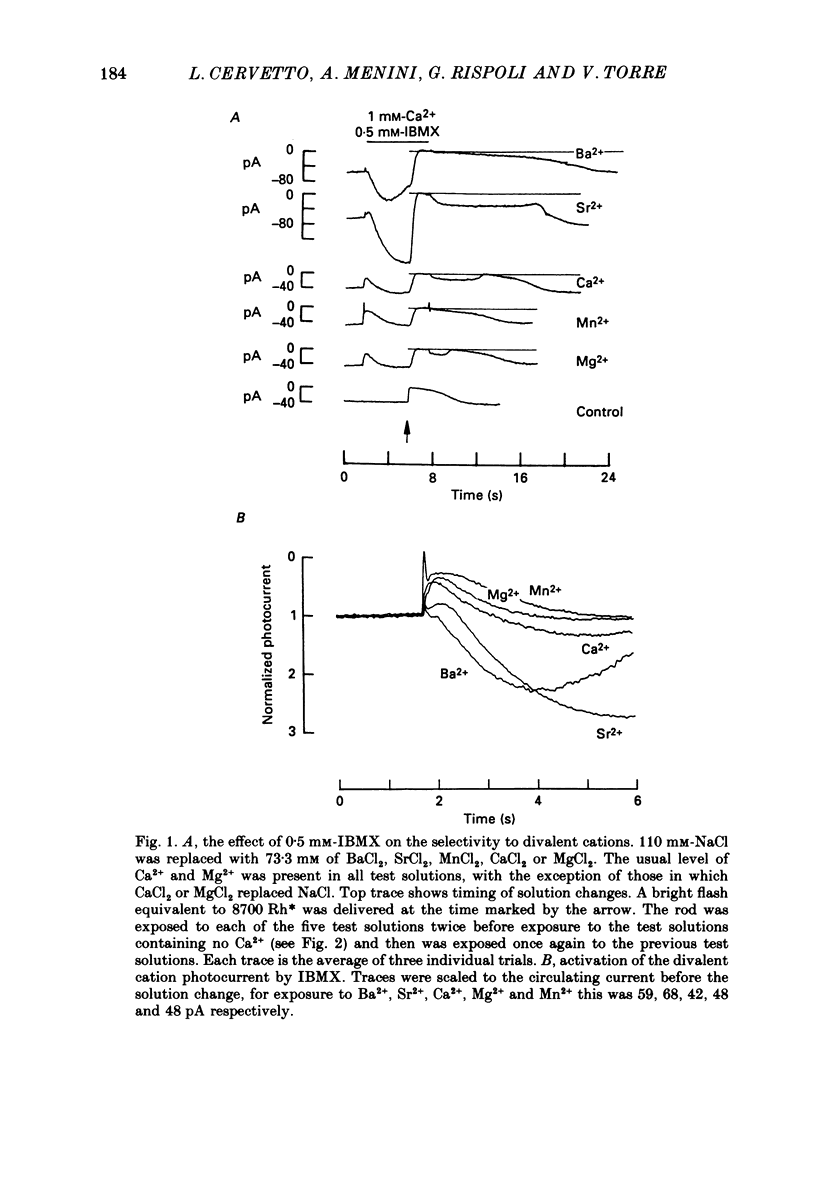
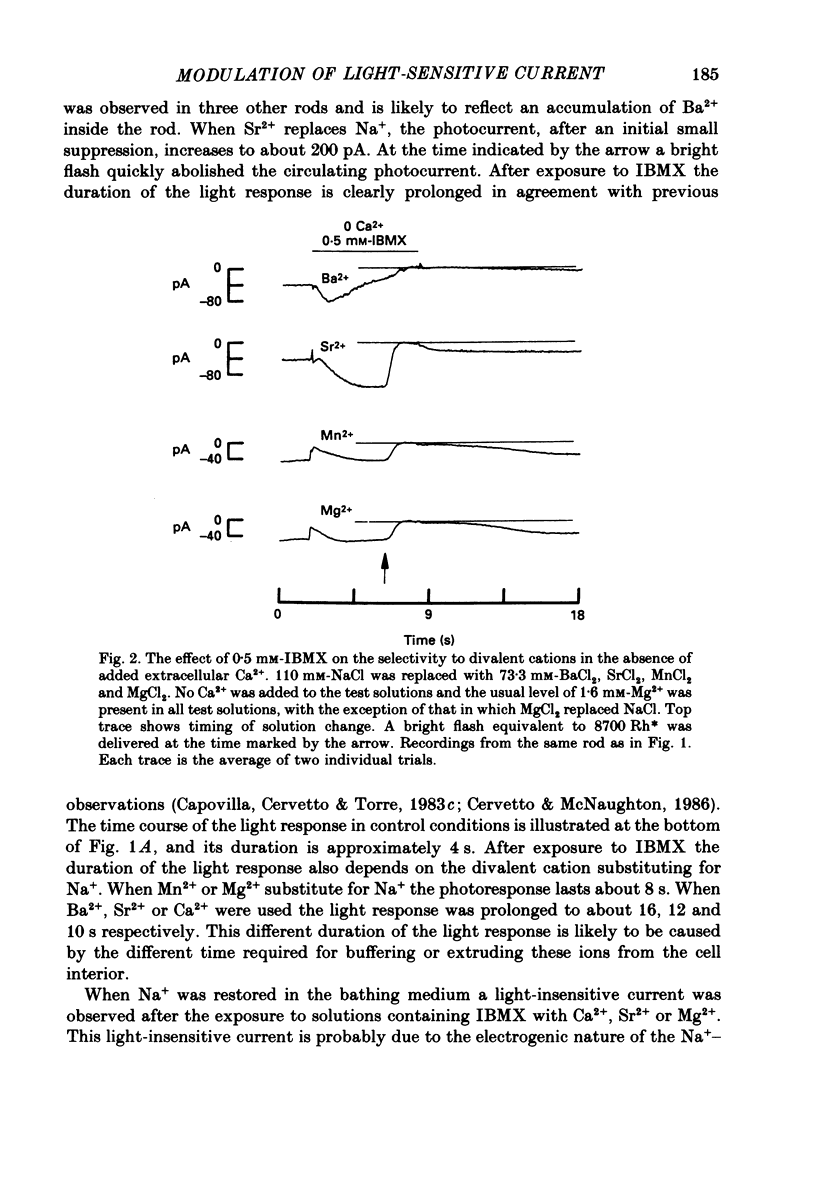
1. By using the method of Hodgkin, McNaughton & Nunn (1985) for rapidly changing the extracellular medium, we analysed the effect of the organic compound IBMX (3-isobutyl-1-methylxanthine) on the movement of divalent cations through the light-sensitive channels of isolated retinal rods of the tiger salamander. 2. When the rod is treated with 0.5 mM-IBMX it is possible to observe photocurrents larger than 50 pA carried by Ba2+, Sr2+, Ca2+, Mg2+ and Mn2+. Under these conditions Ca2+, Mg2+ and Mn2+ carry photocurrents of similar amplitude, while Ba2+ and Sr2+ usually carry larger photocurrents. 3. The movement of Mn2+ through the light-sensitive channel, which is hardly detected under normal conditions, can also be observed after treating the rod for a few seconds with a solution containing 35 mM[Na+]o and 10(-7) M[Ca2+]o. Under these conditions the photocurrent carried by Mn2+ is fully saturated in the presence of 1 mM-extracellular Mn2+. 4. When the rod is pre-treated with an extracellular solution containing 0.5 mM-IBMX the maximal photocurrent which can be carried by 10 mM [Ca2+]o increases from about 10 pA to approximately 200 pA. In these conditions the half-activation of the Ca2+ current is between 1 and 10 mM, that is 20-50 times higher than in normal conditions (Menini, Rispoli & Torre, 1988). 5. When the rod is pre-treated with an extracellular solution containing 0.5 mM-IBMX the half-activation of the photocurrent which can be carried by Mg2+, Ba2+ and Sr2+ is equivalent to or greater than 10 mM. In the absence of pre-treatment with IBMX the half-activation of the photocurrent carried by Mg2+, Ba2+ and Sr2+ is less than 5 mM. 6. We conclude that the light-sensitive channel can exist in at least two distinct open states. The selectivity of the channel in the first open state is as described in a previous paper (Menini et al. 1988). Mn2+, which is hardly permeable through the light-sensitive channel in the first open state, can move through the light-sensitive channel in the second open state. Ca2+, Mg2+, Ba2+ and Sr2+ permeate more freely through the light-sensitive channel in the second open state, probably because the electrostatic interactions between these ions and the channel are less strong.
Full text
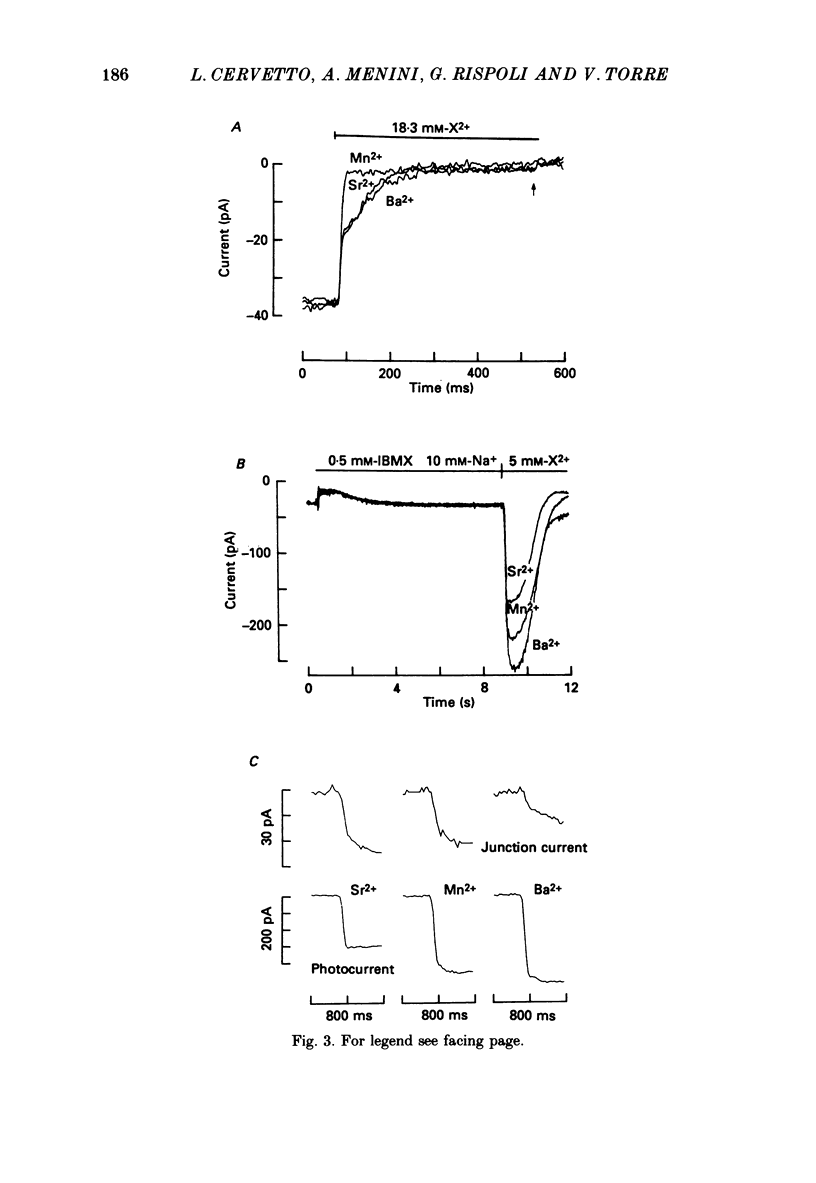
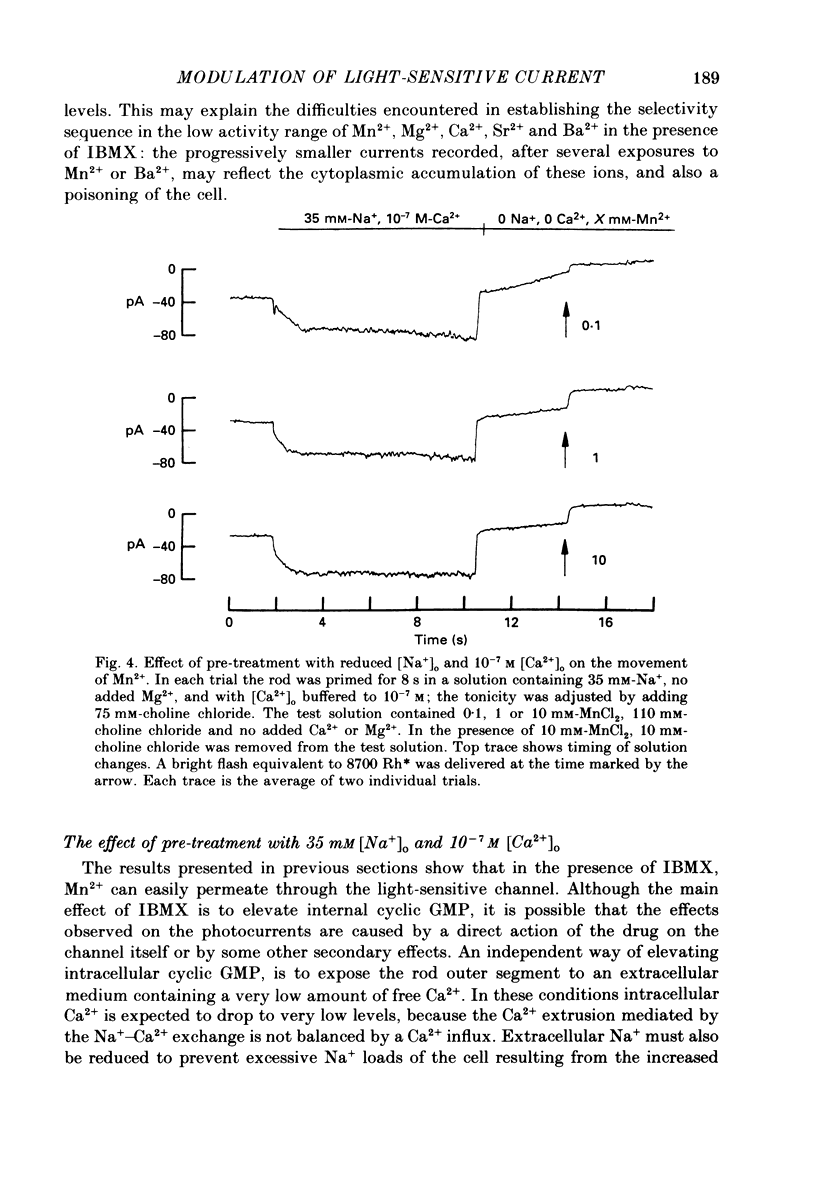
PDF





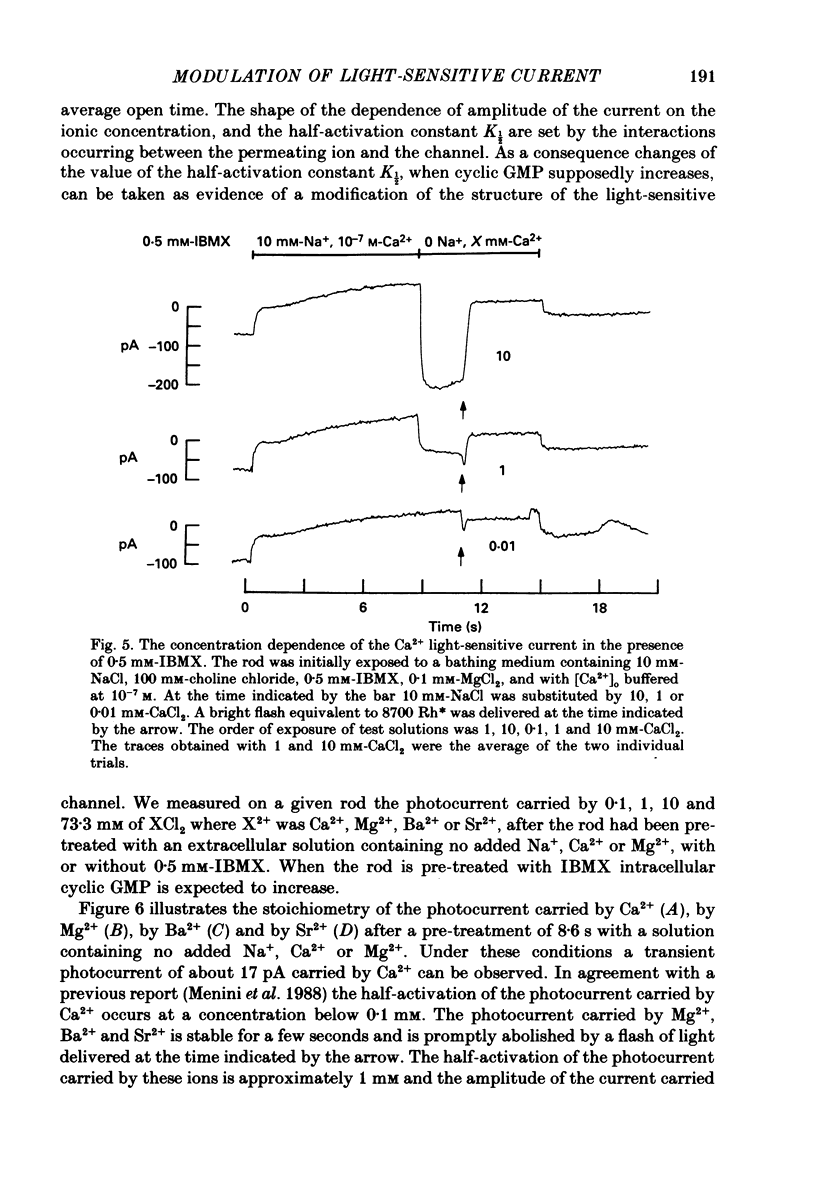




Selected References
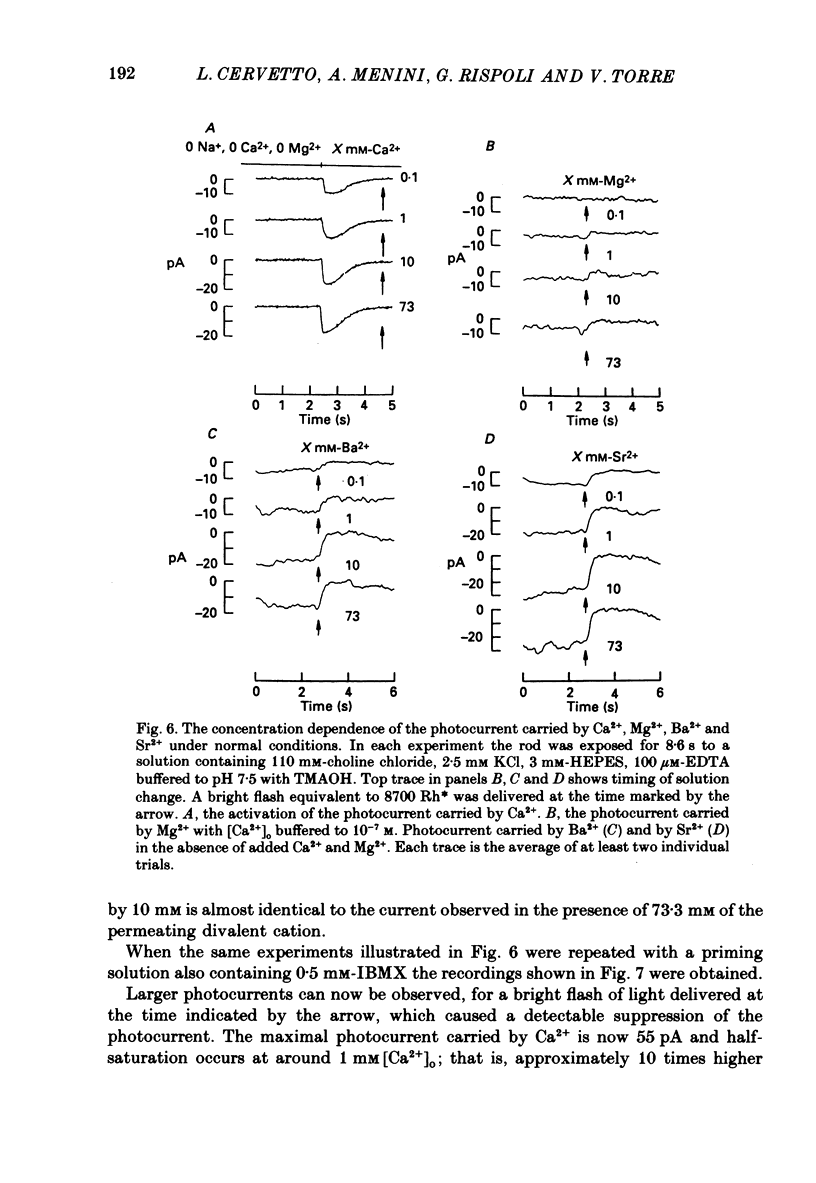
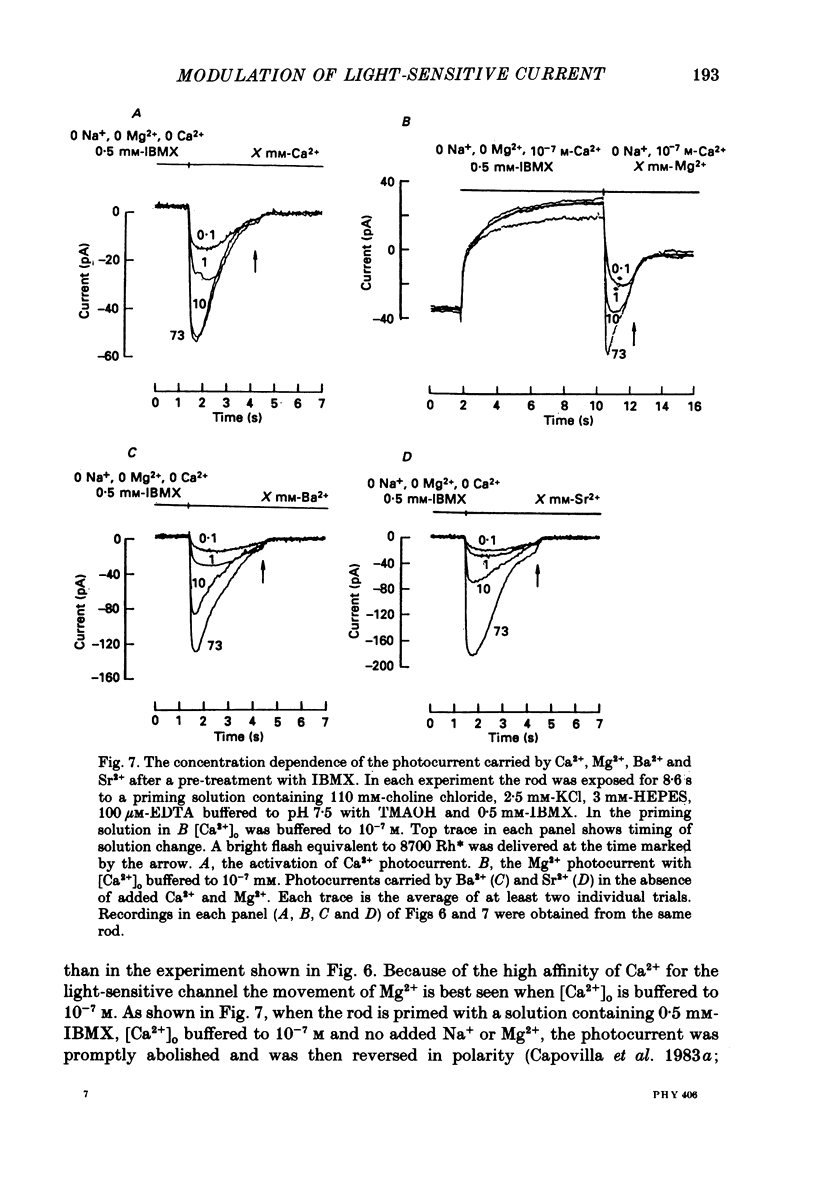
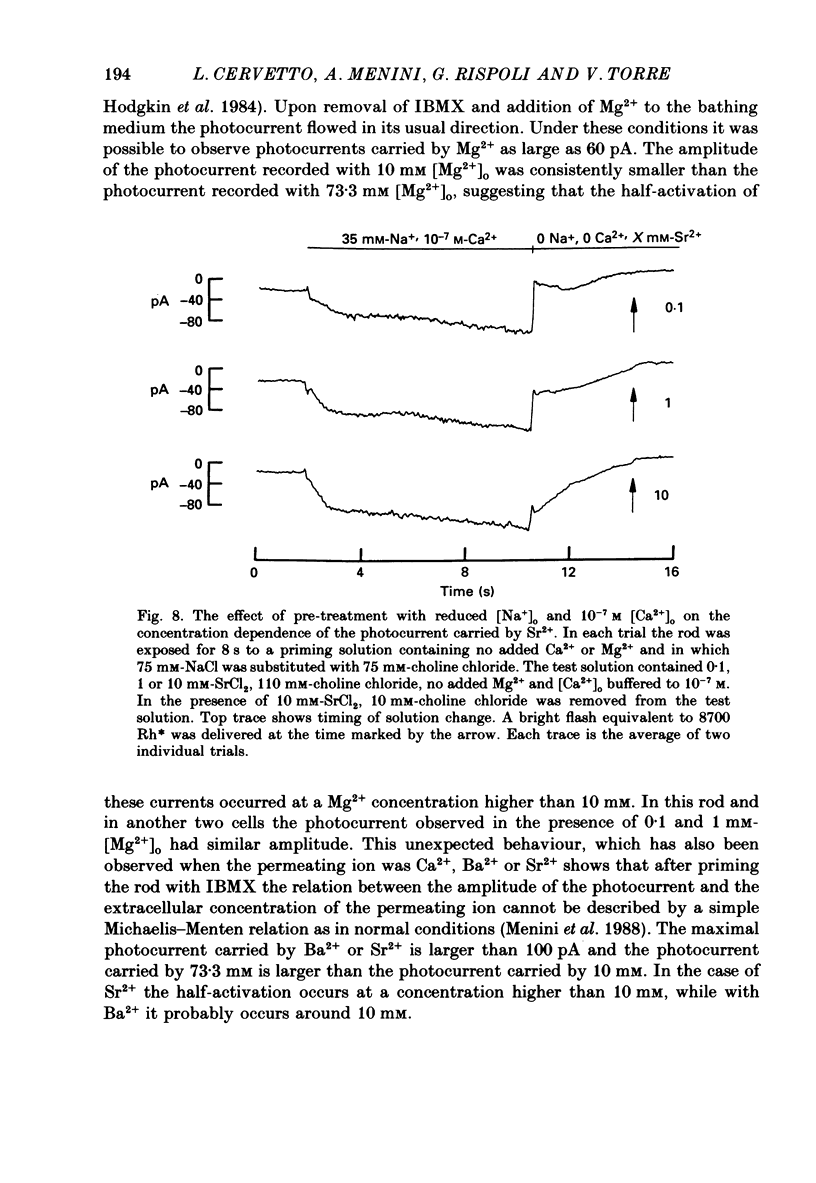
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. Mn ions pass through calcium channels. A possible explanation. J Gen Physiol. 1983 Jun;81(6):805–827. doi: 10.1085/jgp.81.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. The effect of phosphodiesterase inhibitors on the electrical activity of toad rods. J Physiol. 1983 Oct;343:277–294. doi: 10.1113/jphysiol.1983.sp014892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A. Fast ionic flux activated by cyclic GMP in the membrane of cattle rod outer segments. Eur J Biochem. 1983 Apr 15;132(1):1–8. doi: 10.1111/j.1432-1033.1983.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. I., Hall I. A., Ferrendelli J. A. Calcium and cyclic nucleotide regulation in incubated mouse retinas. J Gen Physiol. 1978 May;71(5):595–612. doi: 10.1085/jgp.71.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K., Miyamoto E. Multiple cyclic nucleotide phosphodiesterase activities from rat tissues and occurrence of a calcium-plus-magnesium-ion-dependent phosphodiesterase and its protein activator. Biochem J. 1975 Jan;146(1):109–120. doi: 10.1042/bj1460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N., Fletcher R. T., Chader G. J., Krishna G. Characterization of guanylate cyclase of rod outer segments of the bovine retina. Biochim Biophys Acta. 1978 Apr 12;523(2):506–515. doi: 10.1016/0005-2744(78)90053-0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Matesic D., Liebman P. A. cGMP-dependent cation channel of retinal rod outer segments. Nature. 1987 Apr 9;326(6113):600–603. doi: 10.1038/326600a0. [DOI] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Properties of ion channels closed by light and opened by guanosine 3',5'-cyclic monophosphate in toad retinal rods. J Physiol. 1987 Aug;389:691–715. doi: 10.1113/jphysiol.1987.sp016678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Menini A., Rispoli G., Torre V. The ionic selectivity of the light-sensitive current in isolated rods of the tiger salamander. J Physiol. 1988 Aug;402:279–300. doi: 10.1113/jphysiol.1988.sp017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R. Manganese action potentials in mammalian cardiac muscle. Experientia. 1975 Sep 15;31(9):1048–1049. doi: 10.1007/BF02326952. [DOI] [PubMed] [Google Scholar]
- Pepe I. M., Panfoli I., Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+. FEBS Lett. 1986 Jul 14;203(1):73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Kawamura S., Abramson B., Bownds M. D. Control of the cyclic GMP phosphodiesterase of frog photoreceptor membranes. J Gen Physiol. 1980 Nov;76(5):631–645. doi: 10.1085/jgp.76.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Torre V., Pasino E., Capovilla M., Cervetto L. Rod photoresponses in the absence of external sodium in retinae treated with phosphodiesterase inhibitors. Exp Brain Res. 1981;44(4):427–430. doi: 10.1007/BF00238835. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Wu Y. J., Baird C. E., Hardman J. G. Phosphodiesterases from porcine coronary arteries: inhibition of separated forms by xanthines, papaverine, and cyclic nucleotides. Mol Pharmacol. 1975 Nov;11(6):775–783. [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


