Abstract
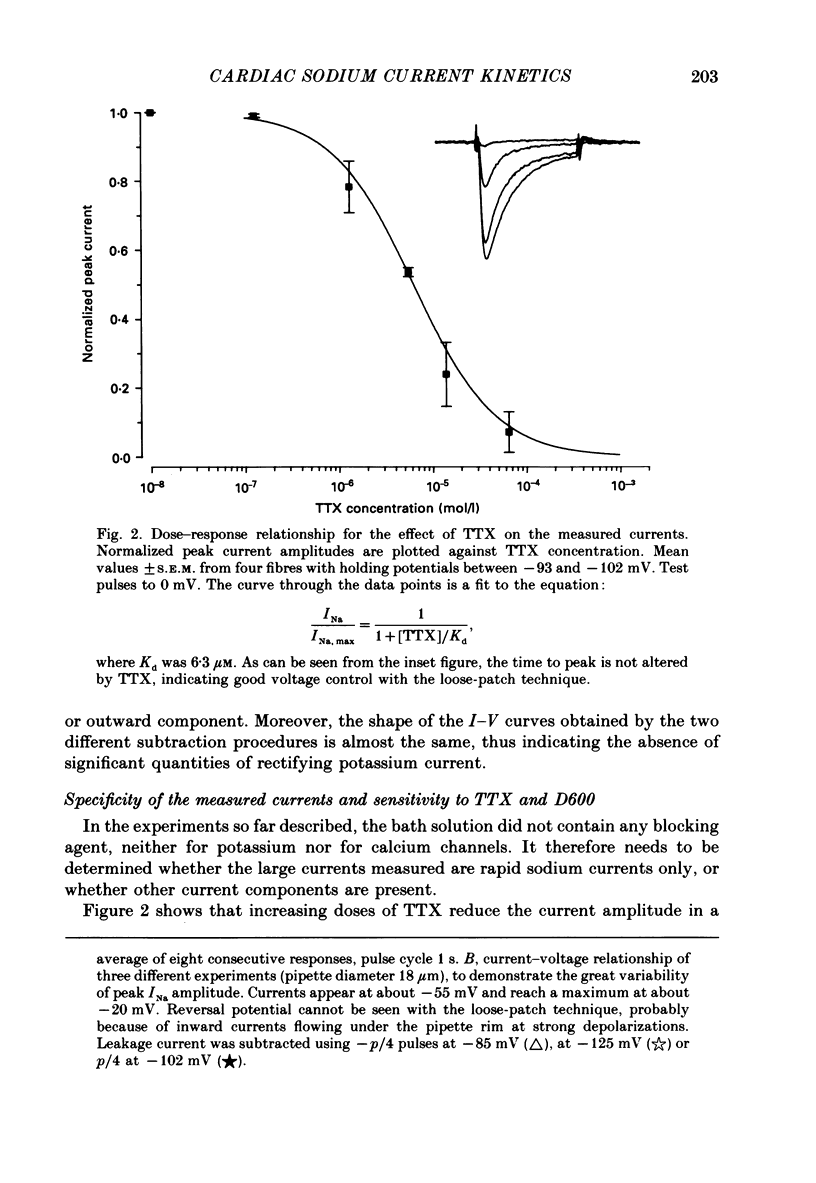
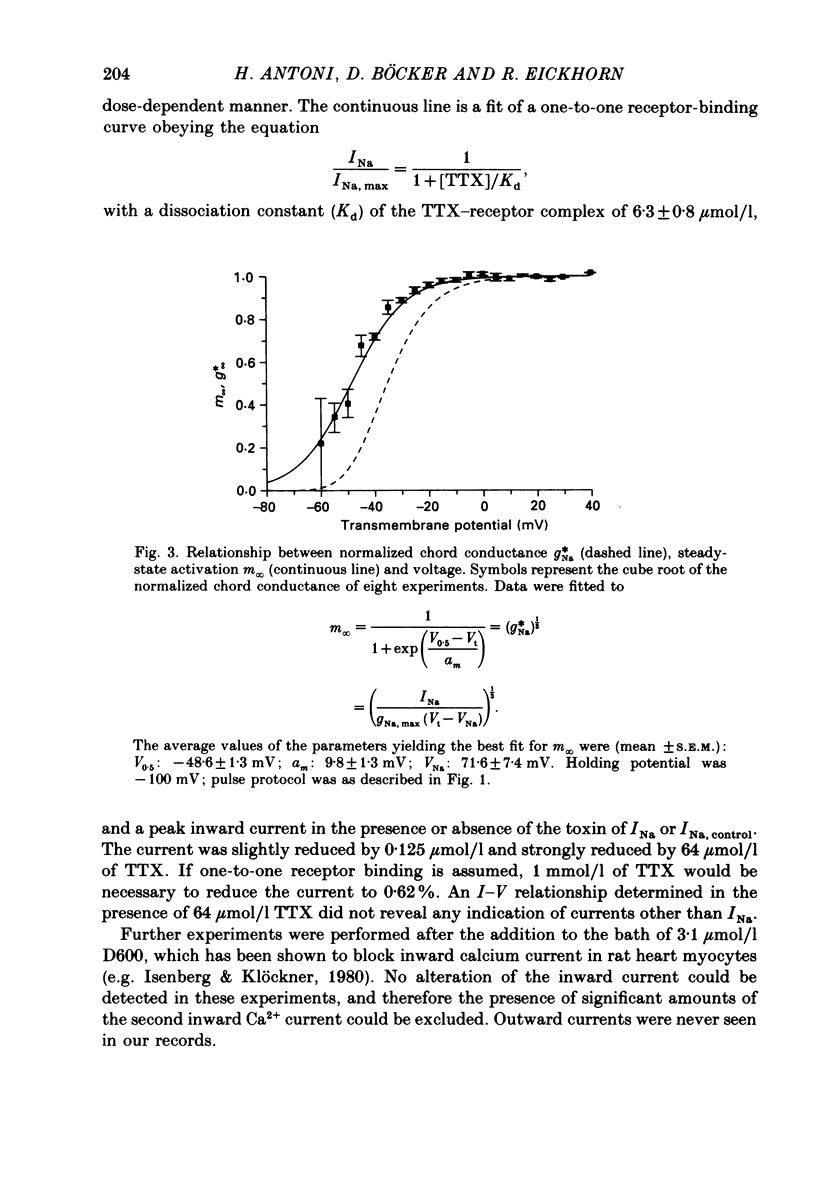
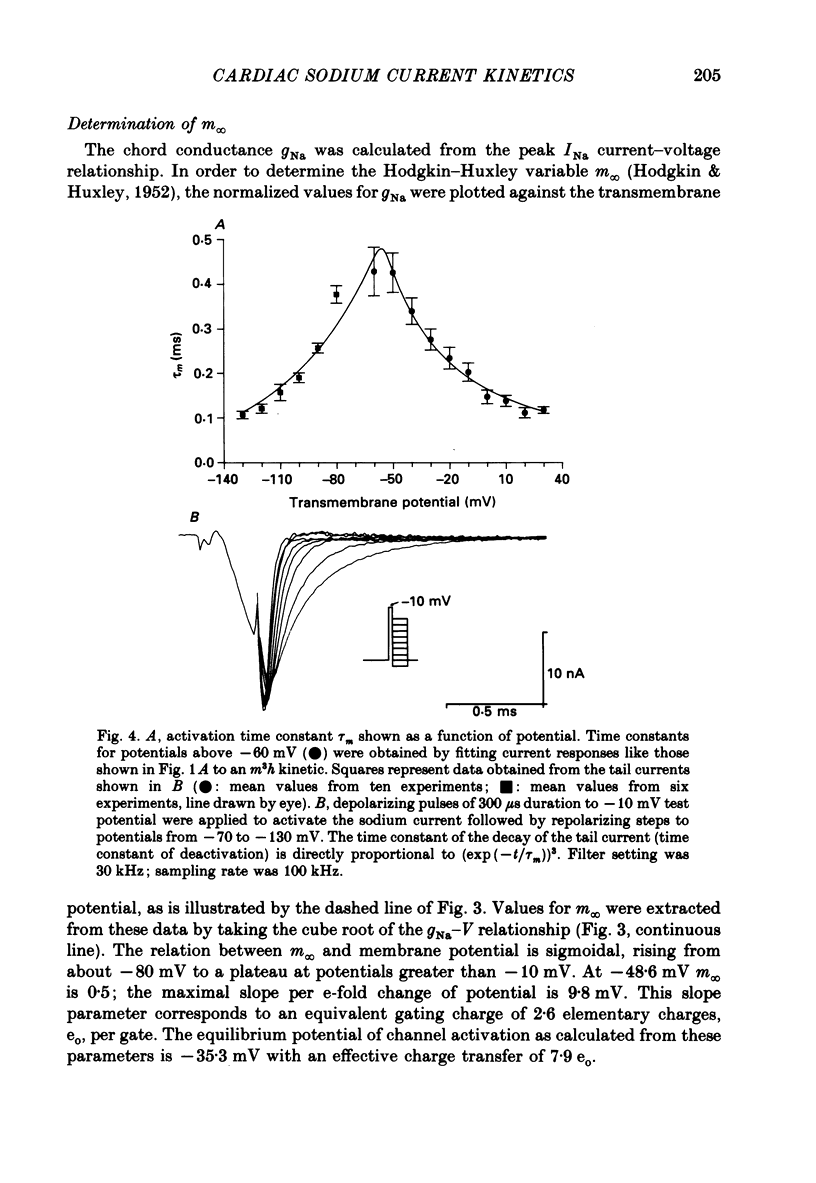
1. Rapid inward sodium current (INa) was studied on intact rat papillary muscles and trabeculae excised from right or left ventricle using the loose-patch-clamp technique. All experiments were carried out at 25 degrees C. 2. Currents were recorded from patches with a large current density of mean 5.9 +/- 0.5 mA/cm2. 3. The current was reduced by tetrodotoxin (TTX) in a dose-dependent manner. The concentration of TTX producing half-maximal blockade of INa was 6.3 +/- 0.8 mumol/l. 4. Na+ current appeared upon depolarization at a threshold potential of about -55 mV and reached its maximum at about -20 mV. 5. Kinetic data were evaluated using the Hodgkin-Huxley model. 6. Time constants of activation (tau m) were estimated using single-pulse and tail-current measurements. They had a maximum of about 0.4 ms near the threshold potential and declined at more positive and at more negative potentials to values near 0.1 ms. 7. Two time constants were necessary to describe inactivation. Both time constants had their maximal values of 135 +/- 8.1 and 29.1 +/- 5.9 ms at about -80 mV and decreased towards 4 and 0.5 ms at potentials positive to -20 mV.
Full text
PDF


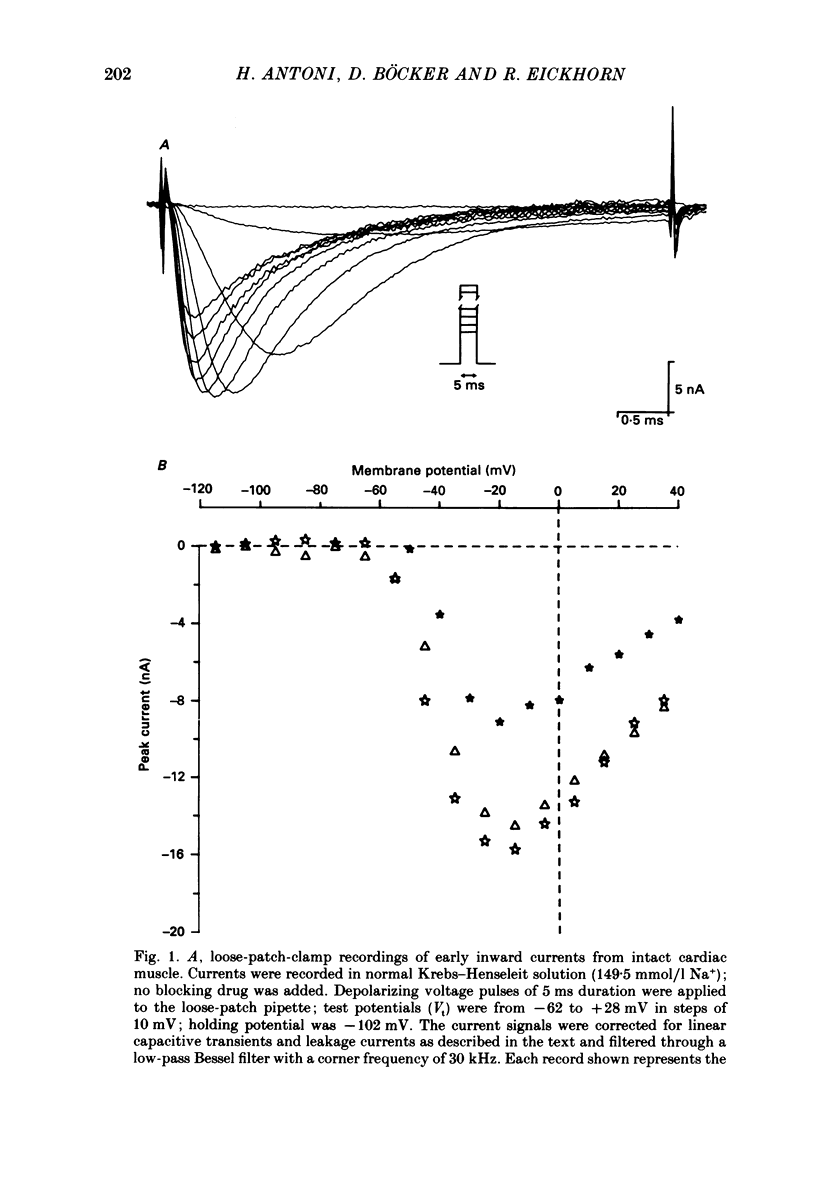





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
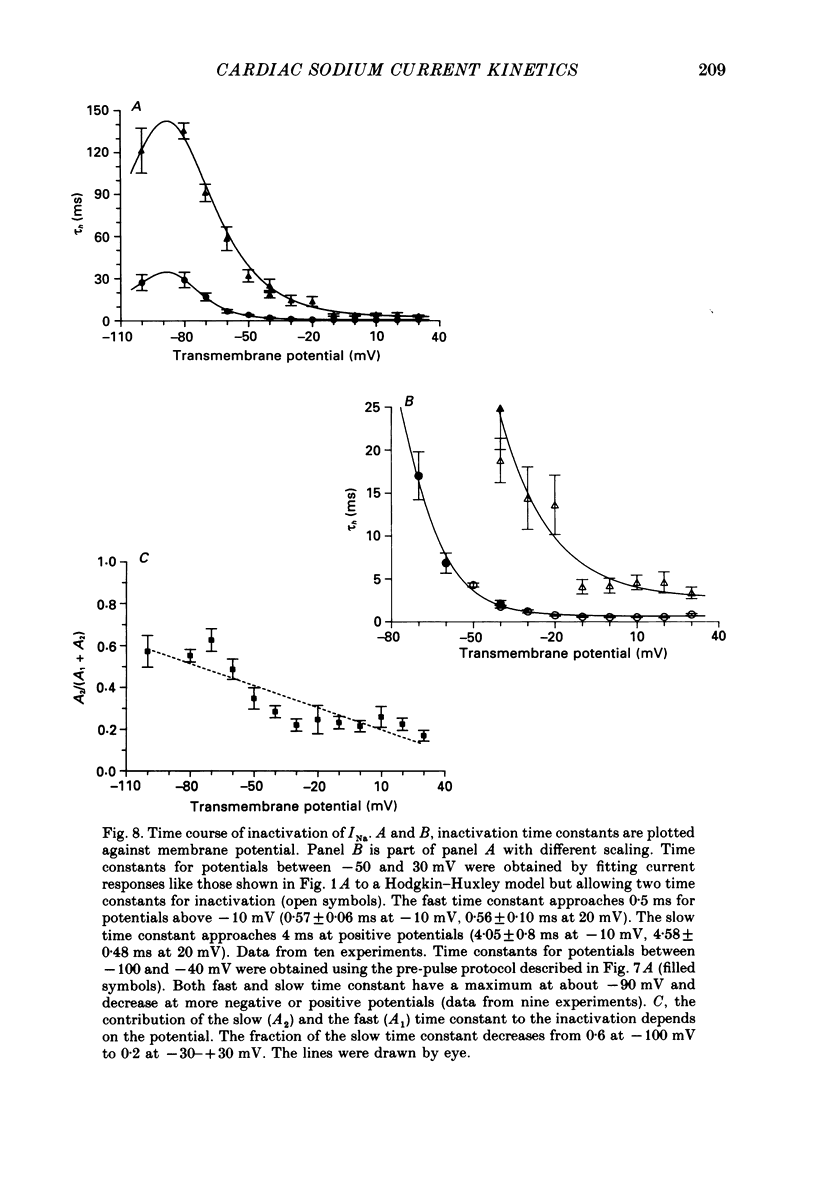
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., McGuigan J. A. Voltage clamping of multicellular myocardial preparations: capabilities and limitations of existing methods. Prog Biophys Mol Biol. 1978;34(3):219–254. doi: 10.1016/0079-6107(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Benndorf K., Nilius B. Inactivation of sodium channels in isolated myocardial mouse cells. Eur Biophys J. 1987;15(2):117–127. doi: 10.1007/BF00257505. [DOI] [PubMed] [Google Scholar]
- Bodewei R., Hering S., Lemke B., Rosenshtraukh L. V., Undrovinas A. I., Wollenberger A. Characterization of the fast sodium current in isolated rat myocardial cells: simulation of the clamped membrane potential. J Physiol. 1982 Apr;325:301–315. doi: 10.1113/jphysiol.1982.sp014151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky J. J., Tsien R. W. Sodium channels in rabbit cardiac Purkinje fibres. Nature. 1979 Mar 15;278(5701):265–268. doi: 10.1038/278265a0. [DOI] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follmer C. H., ten Eick R. E., Yeh J. Z. Sodium current kinetics in cat atrial myocytes. J Physiol. 1987 Mar;384:169–197. doi: 10.1113/jphysiol.1987.sp016449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Beeler G. W., Jr The voltage clamp and cardiac electrophysiology. Circ Res. 1975 Oct;37(4):403–413. doi: 10.1161/01.res.37.4.403. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., January C. T., Makielski J. C. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985 Apr;56(4):475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Catterall W. A. Inhibition of sodium channels by D600. Mol Pharmacol. 1979 Jan;15(1):174–178. [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Bonilla E., Casadei J., Barchi R. Immunocytochemical localization of the mammalian voltage-dependent sodium channel using polyclonal antibodies against the purified protein. J Neurosci. 1984 Sep;4(9):2259–2268. doi: 10.1523/JNEUROSCI.04-09-02259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Electrical activity and contraction in cells isolated from rat and guinea-pig ventricular muscle: a comparative study. J Physiol. 1987 Oct;391:527–544. doi: 10.1113/jphysiol.1987.sp016754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Almers W. Photobleaching through glass micropipettes: sodium channels without lateral mobility in the sarcolemma of frog skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):946–950. doi: 10.1073/pnas.79.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Kunze D. L., Brown A. M. Effects of dihydropyridine calcium channel modulators on cardiac sodium channels. Am J Physiol. 1988 Jan;254(1 Pt 2):H140–H147. doi: 10.1152/ajpheart.1988.254.1.H140. [DOI] [PubMed] [Google Scholar]


