Abstract
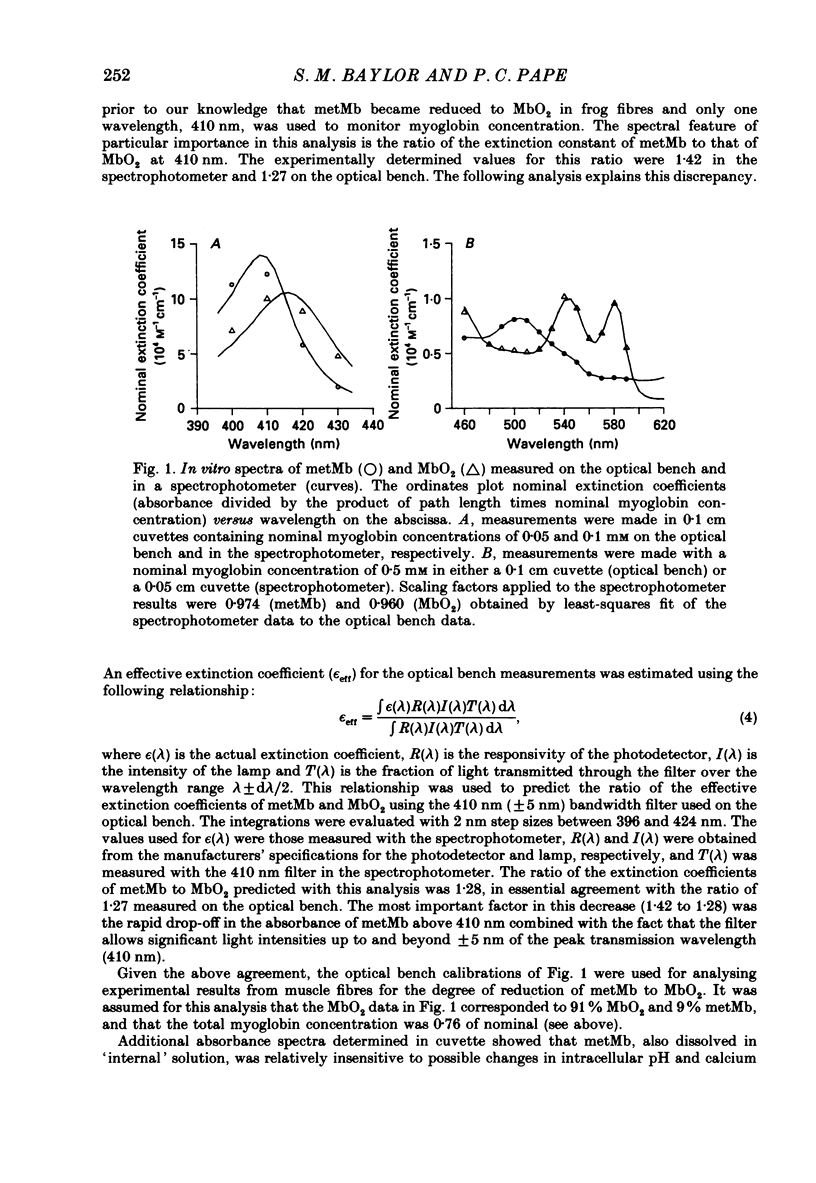
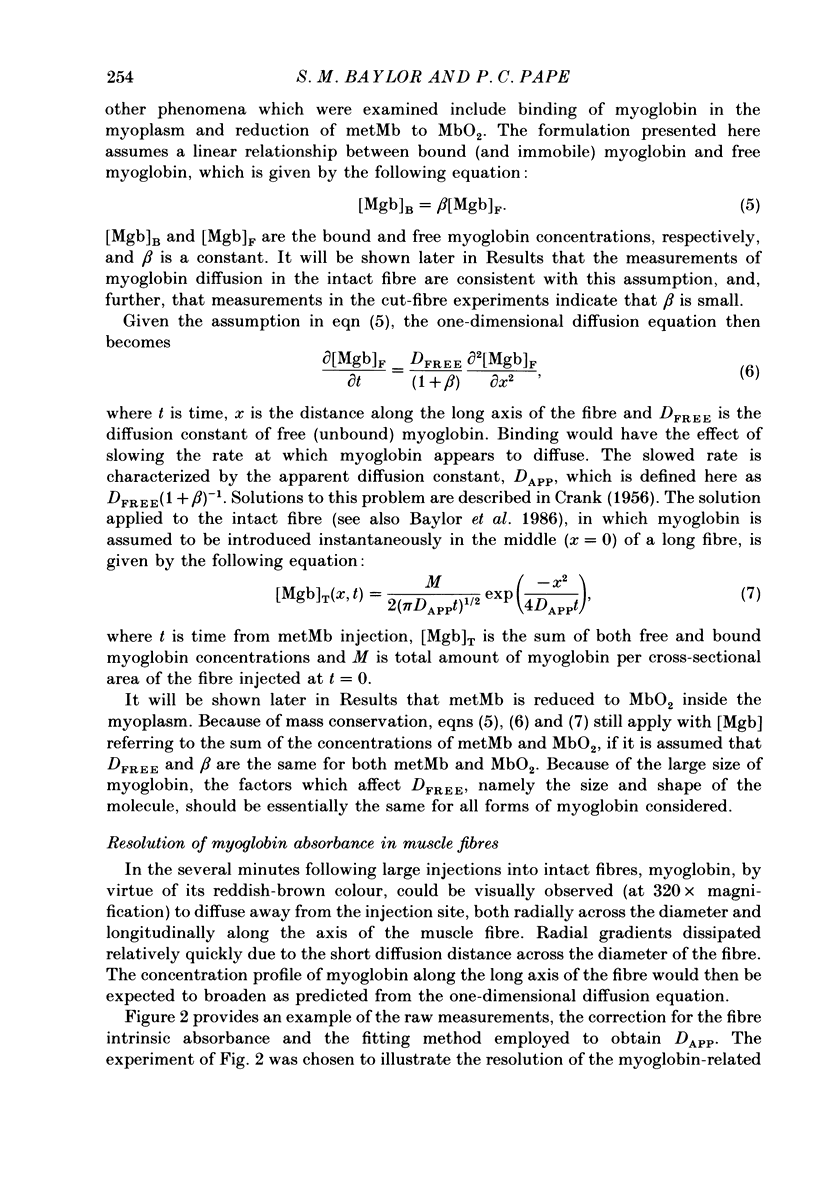
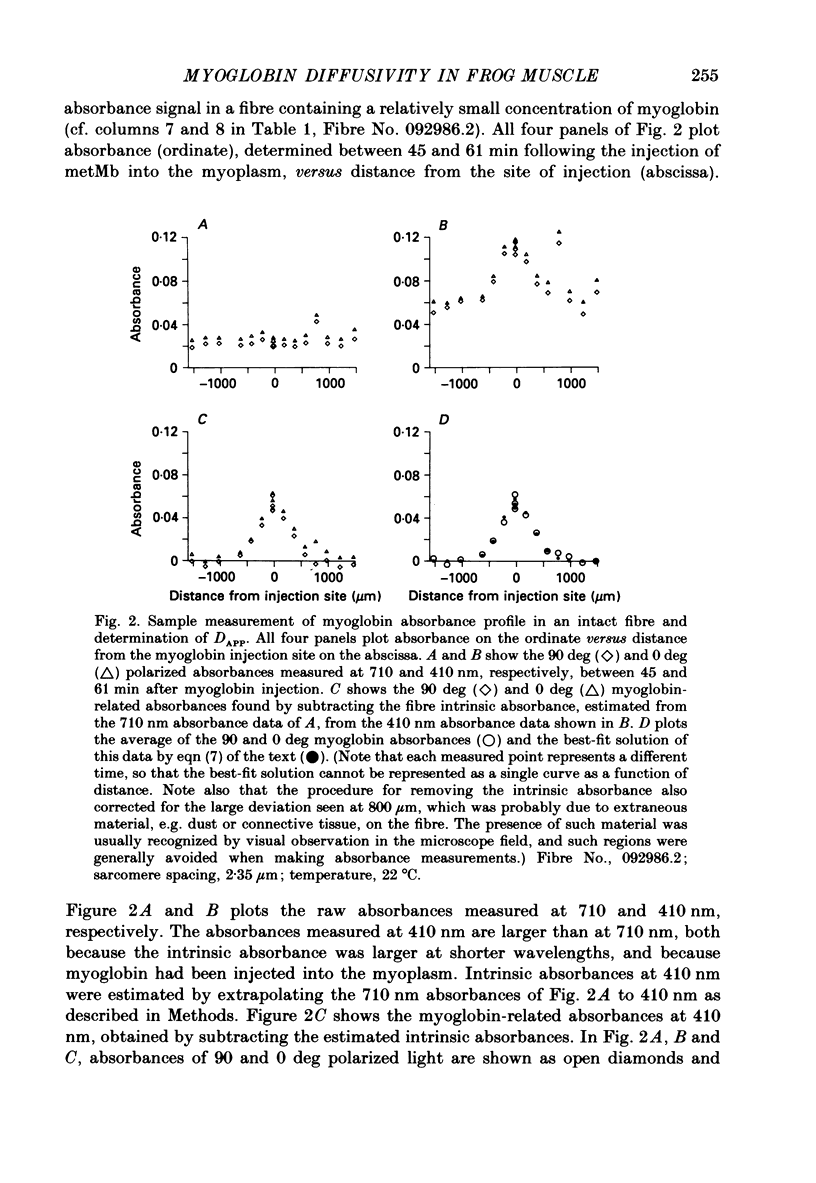
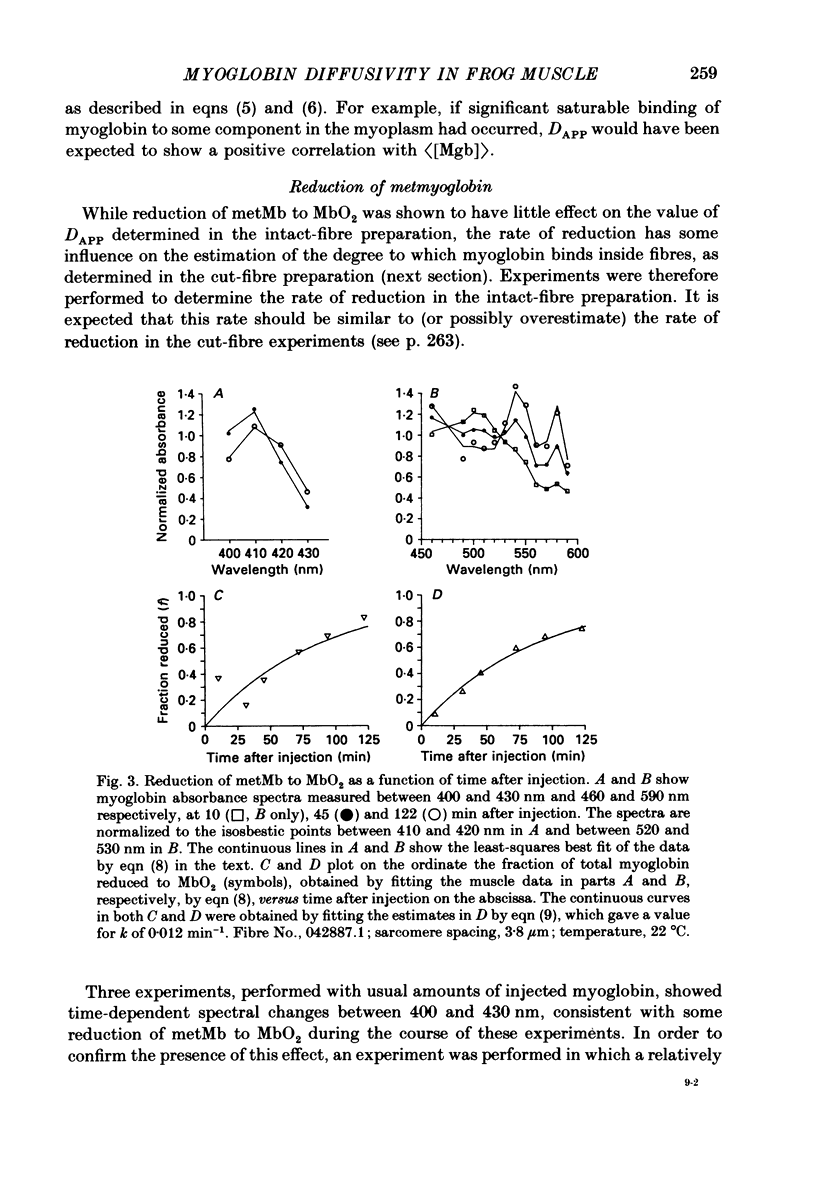
1. Experiments were carried out on intact, single skeletal muscle fibres from frog in order to estimate the apparent diffusion constant of myoglobin (denoted DAPP) in the myoplasm of living muscle cells. An optical technique was employed to measure myoglobin concentration along the fibre axis following injection of metmyoglobin (denoted metMb) at a point source. The concentration profiles were fitted by the one-dimensional diffusion equation to give estimates of DAPP. The method relied on the fact that myoglobin is normally absent from these frog fibres, thus permitting resolution of the myoglobin-related absorbance above the intrinsic absorbance of the fibre. 2. One complication in the method was that metMb became significantly reduced to oxymyoglobin (denoted MbO2) during the elapsed time before measurement of the concentration profile. The rate of reduction was evaluated by fitting myoglobin-related absorbance spectra, measured at different times following injection of metMb, with in vitro absorbance spectra of metMb and MbO2. Results from four experiments indicated that reduction could be described by a first-order, irreversible reaction having an average rate constant of 0.0164 min-1 (22 degrees C). The effect of reduction on the fitting of DAPP was taken into account. 3. DAPP was determined under three fibre conditions: (1) long sarcomere spacing (3.6-3.8 microns) at 16 degrees C, (2) long sarcomere spacing at 22 degrees C, and (3) normal sarcomere spacing (2.4-2.7 microns) at 22 degrees C. The average values for DAPP under these conditions were: (1) 0.12 (n = 5); (2) 0.17 (n = 5); and (3) 0.15 (n = 7) x 10(-6) cm2 s-1. The average value at 22 degrees C, 0.16 x 10(-6) cm2 s-1, is about 4 times smaller than values for myoglobin diffusivity at 20 degrees C commonly assumed in models of facilitated transport of oxygen by myoglobin. 4. In order to test the possibility that the unexpectedly low value of DAPP found in intact fibres might be due to the binding of myoglobin to relatively immobile sites in myoplasm, experiments were carried out in a cut-fibre preparation using a technique described by Maylie, Irving, Sizto & Chandler (1987 b) for determining the diffusion constants and degree of myoplasmic binding of absorbance dyes. Values for DAPP and the factor (denoted 1 + beta) by which the total myoglobin concentration exceeded the free myoglobin concentration were obtained by fitting the absorbance data by solutions of the one-dimensional diffusion equation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G., Rose R. M. Proceedings: Aequorin-light and tension responses from bundles of myofibrils following a sudden change in free calcium. J Physiol. 1974 Sep;241(2):104P–106P. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Hui C. S., Quinta-Ferreira M. E. Properties of the metallochromic dyes Arsenazo III, Antipyrylazo III and Azo1 in frog skeletal muscle fibres at rest. J Physiol. 1986 Aug;377:89–141. doi: 10.1113/jphysiol.1986.sp016178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantino M., Squire J. Resting myosin cross-bridge configuration in frog muscle thick filaments. J Cell Biol. 1986 Feb;102(2):610–618. doi: 10.1083/jcb.102.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. P. Myoglobin function in exercising skeletal muscle. Science. 1982 Apr 30;216(4545):523–525. doi: 10.1126/science.7071598. [DOI] [PubMed] [Google Scholar]
- Cole R. P., Wittenberg B. A., Caldwell P. R. Myoglobin function in the isolated fluorocarbon-perfused dog heart. Am J Physiol. 1978 May;234(5):H567–H572. doi: 10.1152/ajpheart.1978.234.5.H567. [DOI] [PubMed] [Google Scholar]
- Federspiel W. J. A model study of intracellular oxygen gradients in a myoglobin-containing skeletal muscle fiber. Biophys J. 1986 Apr;49(4):857–868. doi: 10.1016/S0006-3495(86)83715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. E. On facilitated oxygen diffusion in muscle tissues. Biophys J. 1980 Mar;29(3):437–458. doi: 10.1016/S0006-3495(80)85145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROTE J., THEWS G. [Requirements for the oxygen supply of heart muscle tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1962;276:142–165. [PubMed] [Google Scholar]
- Gayeski T. E., Honig C. R. Direct measurement of intracellular O2 gradients; role of convection and myoglobin. Adv Exp Med Biol. 1983;159:613–621. doi: 10.1007/978-1-4684-7790-0_54. [DOI] [PubMed] [Google Scholar]
- Hagler L., Coppes R. I., Jr, Herman R. H. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J Biol Chem. 1979 Jul 25;254(14):6505–6514. [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. P., Kennedy F. G. Intracellular O2 gradients in cardiac myocytes. Lack of a role for myoglobin in facilitation of intracellular O2 diffusion. Biochem Biophys Res Commun. 1982 Mar 30;105(2):419–424. doi: 10.1016/0006-291x(82)91450-4. [DOI] [PubMed] [Google Scholar]
- Kreuzer F. Facilitated diffusion of oxygen and its possible significance; a review. Respir Physiol. 1970 Apr;9(1):1–30. doi: 10.1016/0034-5687(70)90002-2. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Livingston D. J., La Mar G. N., Brown W. D. Myoglobin diffusion in bovine heart muscle. Science. 1983 Apr 1;220(4592):71–73. doi: 10.1126/science.6828881. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S. The effect of myoglobin-facilitated oxygen transport on the basal metabolism of papillary muscle. Biophys J. 1987 Jun;51(6):905–913. doi: 10.1016/S0006-3495(87)83418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Boyarsky G., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing tetramethylmurexide. J Gen Physiol. 1987 Jan;89(1):145–176. doi: 10.1085/jgp.89.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll W. The diffusion coefficient of myoglobin in muscle homogenate. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(3):247–251. doi: 10.1007/BF00362587. [DOI] [PubMed] [Google Scholar]
- Murray J. D. On the role of myoglobin in muscle respiration. J Theor Biol. 1974 Sep;47(1):115–126. doi: 10.1016/0022-5193(74)90102-7. [DOI] [PubMed] [Google Scholar]
- Naylor G. R., Bartels E. M., Bridgman T. D., Elliott G. F. Donnan potentials in rabbit psoas muscle in rigor. Biophys J. 1985 Jul;48(1):47–59. doi: 10.1016/S0006-3495(85)83759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros-Moreno V., Wittenberg J. B. The self-diffusion coefficients of myoglobin and hemoglobin in concentrated solutions. J Biol Chem. 1972 Feb 10;247(3):895–901. [PubMed] [Google Scholar]
- Salathé E. P., Kolkka R. W. Reduction of anoxia through myoglobin-facilitated diffusion of oxygen. Biophys J. 1986 Nov;50(5):885–894. doi: 10.1016/S0006-3495(86)83529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Oshino N., Chance B., Silver I. A. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys. 1978 Nov;191(1):8–22. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Murray J. D. Effect of the rate of oxygen consumption on muscle respiration. J Math Biol. 1977 Feb 28;4(1):1–20. doi: 10.1007/BF00276348. [DOI] [PubMed] [Google Scholar]
- Taylor D., Hochstein P. Reduction of metmyoglobin in myocytes. J Mol Cell Cardiol. 1982 Mar;14(3):133–140. doi: 10.1016/0022-2828(82)90111-0. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B., Caldwell P. R. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975 Dec 10;250(23):9038–9043. [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7503–7507. doi: 10.1073/pnas.84.21.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem. 1985 Jun 10;260(11):6548–6554. [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I., YOKOTA K. N., SHIKAMA K. PREPARATION OF CRYSTALLINE OXYMYOGLOBIN FROM HORSE HEART. J Biol Chem. 1964 Dec;239:4151–4153. [PubMed] [Google Scholar]


