Abstract
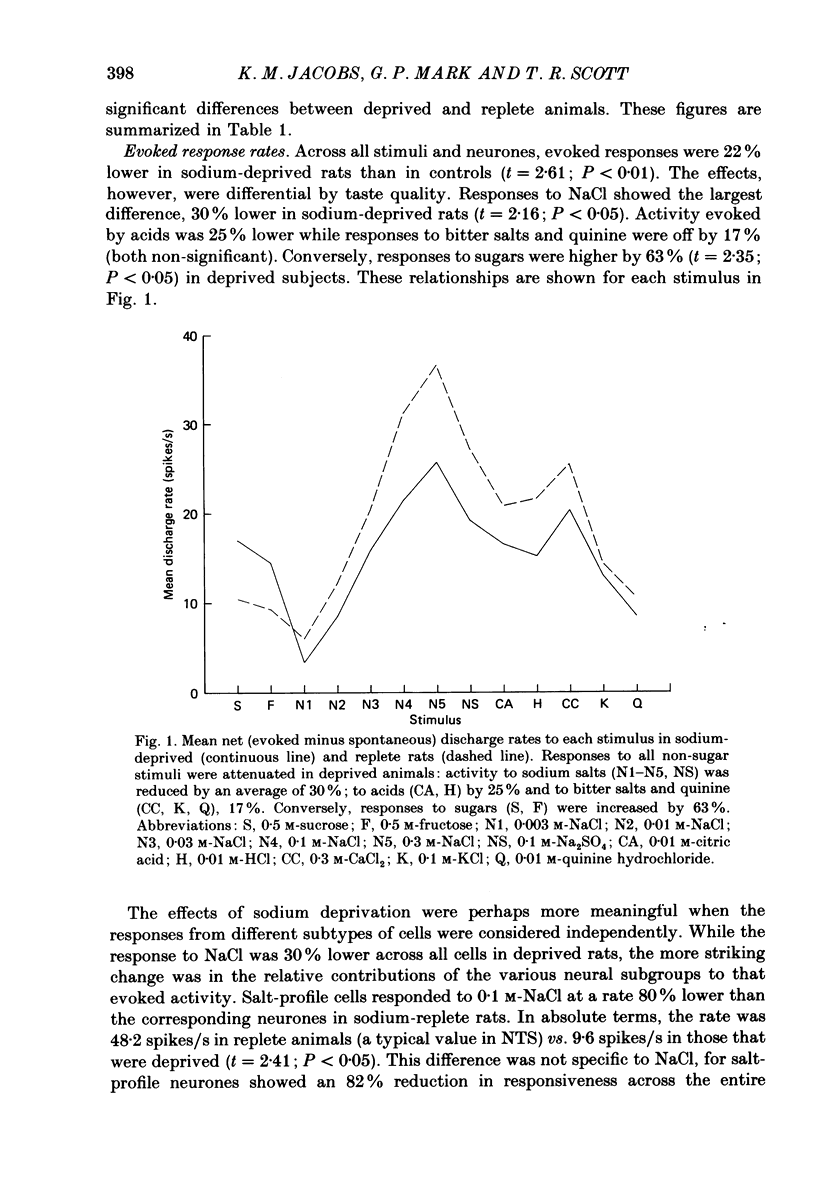
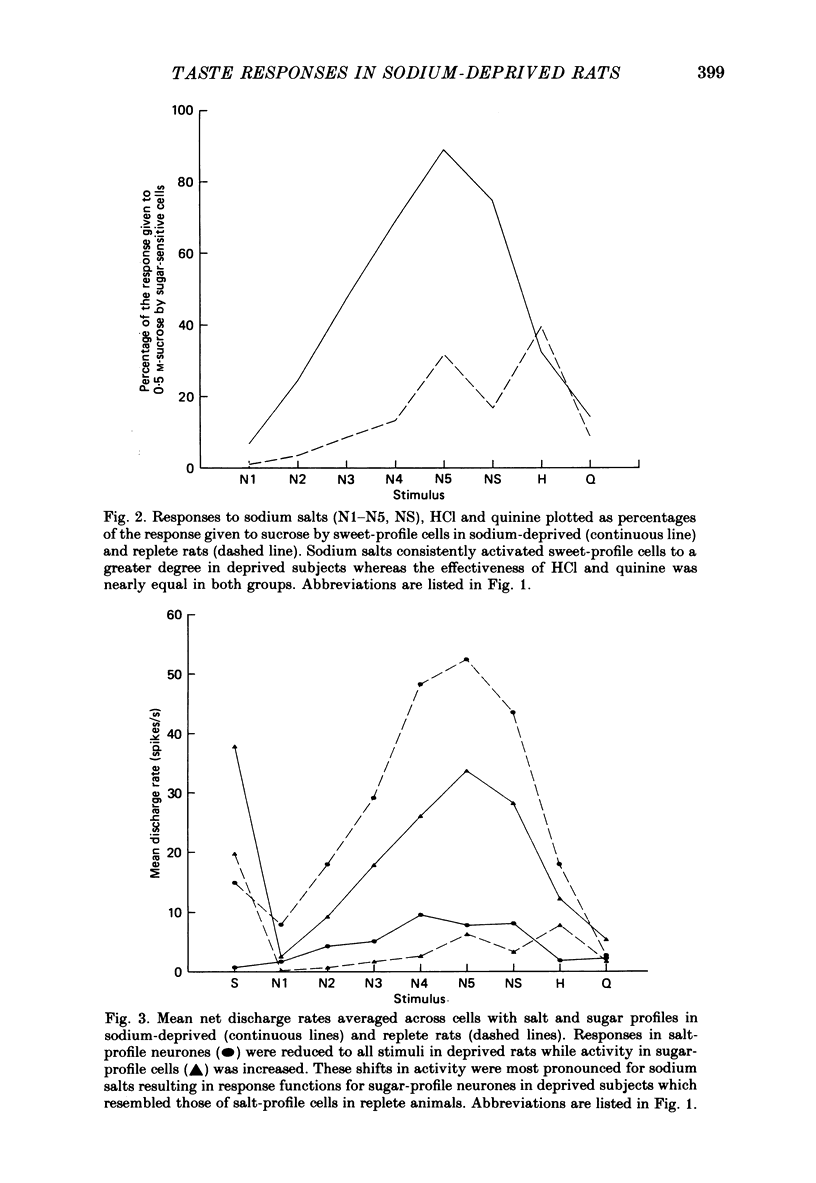
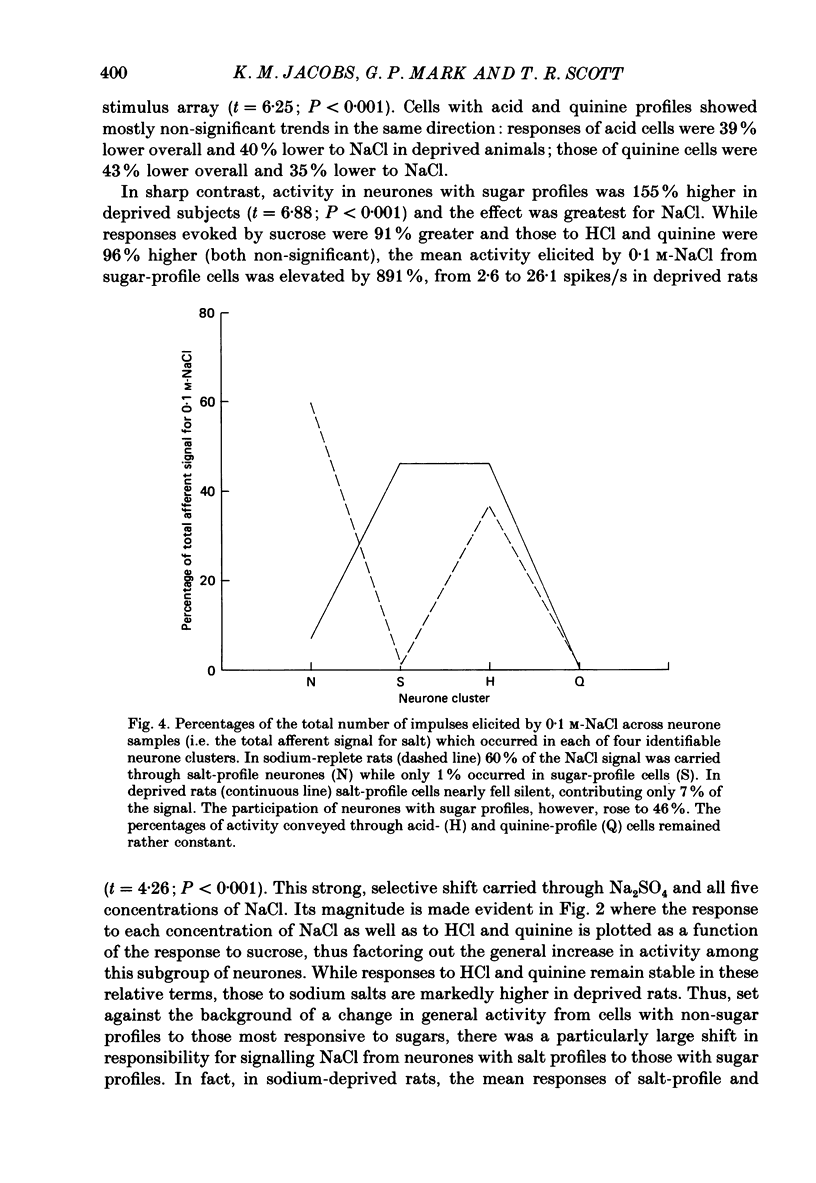
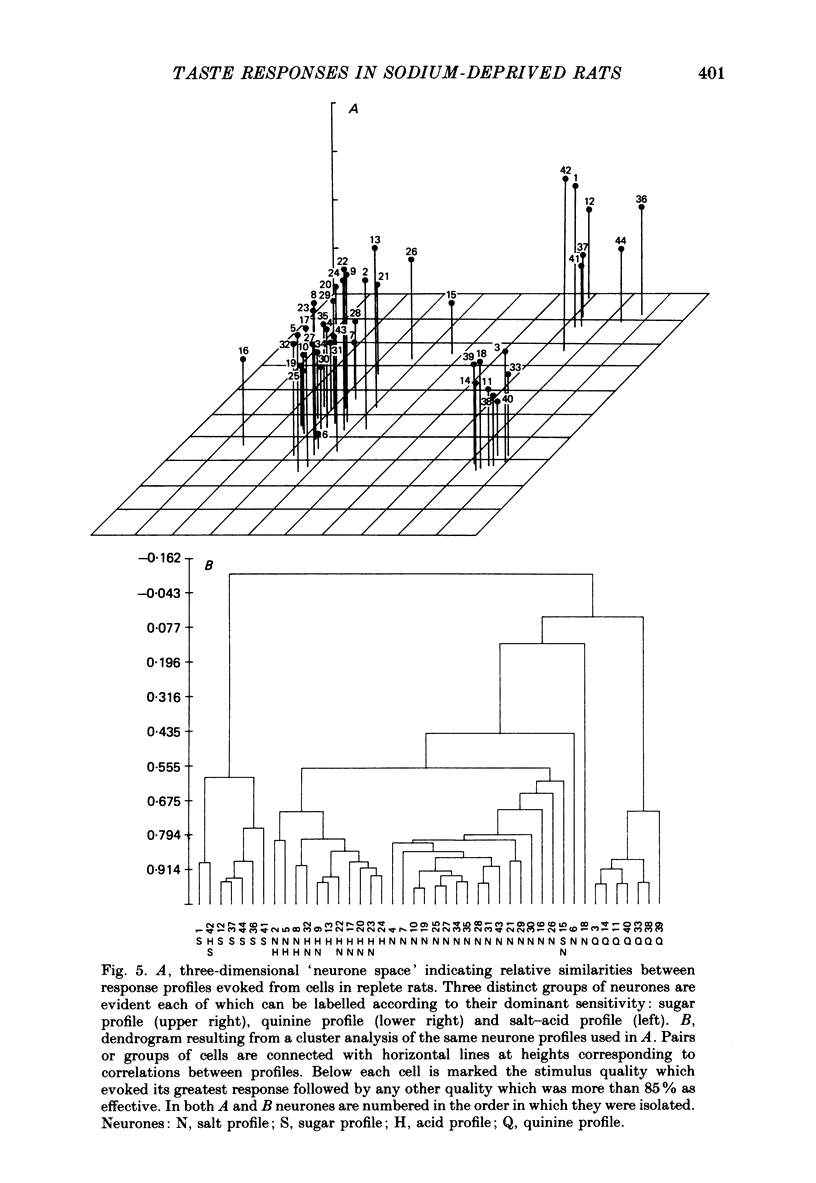
1. Maintenance of sodium balance is crucial to mammals and is expressed in the innate salt appetite. With depletion, sodium preference is exaggerated, hypertonic solutions accepted and salt balance restored. This compensatory behaviour is thought to result from a centrally induced change in taste responsiveness. This proposal was tested by recording taste activity from ninety-four single neurones in the nucleus tractus solitarius of sodium-replete (N = 44) and of deprived (N = 50) rats. Twelve Wistar rats were given a nominally sodium-free diet for 10-13 days, and the resulting sodium depletion confirmed by flame photometry of their urine. Nine rats provided control data. Taste stimuli included five concentrations of NaCl (0.003-0.3 M) plus eight other salts, acids, sugars and alkaloids. 2. Taste responsiveness was generally reduced in sodium-depleted rats. Spontaneous activity was 33% lower while responses to sodium salts lagged by a mean of 30%, to acids by 25% and to bitter salts and quinine by 17%. Mean activity to sugars was 60% higher in the deprived group. 3. Activity in sugar- and salt-profile neurones was most affected. In deprived animals responses to sodium salts were lower by 80% among salt-profile cells while among sugar-profile neurones activity to these stimuli was nearly 10 times greater than in controls. These changes in activity resulted in a dramatic shift in the participation of sodium- and sugar-profile cells in the afferent signal for NaCl. In replete animals 60% of sodium-induced activity was transmitted through salt-profile cells while only 1% occurred in sugar-profile neurones. In deprived subjects this situation was nearly reversed as 7% of the total NaCl response was conveyed through salt-profile cells while the contribution of neurones with sugar-profiles rose to 46%. 4. Multidimensional stimulus spaces based on average activity in each of four identifiable neurone subgroups demonstrated a shift in the affiliation of sodium salts away from bitter and acid stimuli and towards sugars. 5. These results confirm earlier findings from the chorda tympani that sodium deprivation suppresses activity evoked by sodium salts. However the application of more recent analytical procedures permits quite a different interpretation of this finding. The overall decrease is merely the net effect of a shift in the major responsibility for encoding sodium from salt-profile neurones to those whose primary sensitivity is to sugars.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




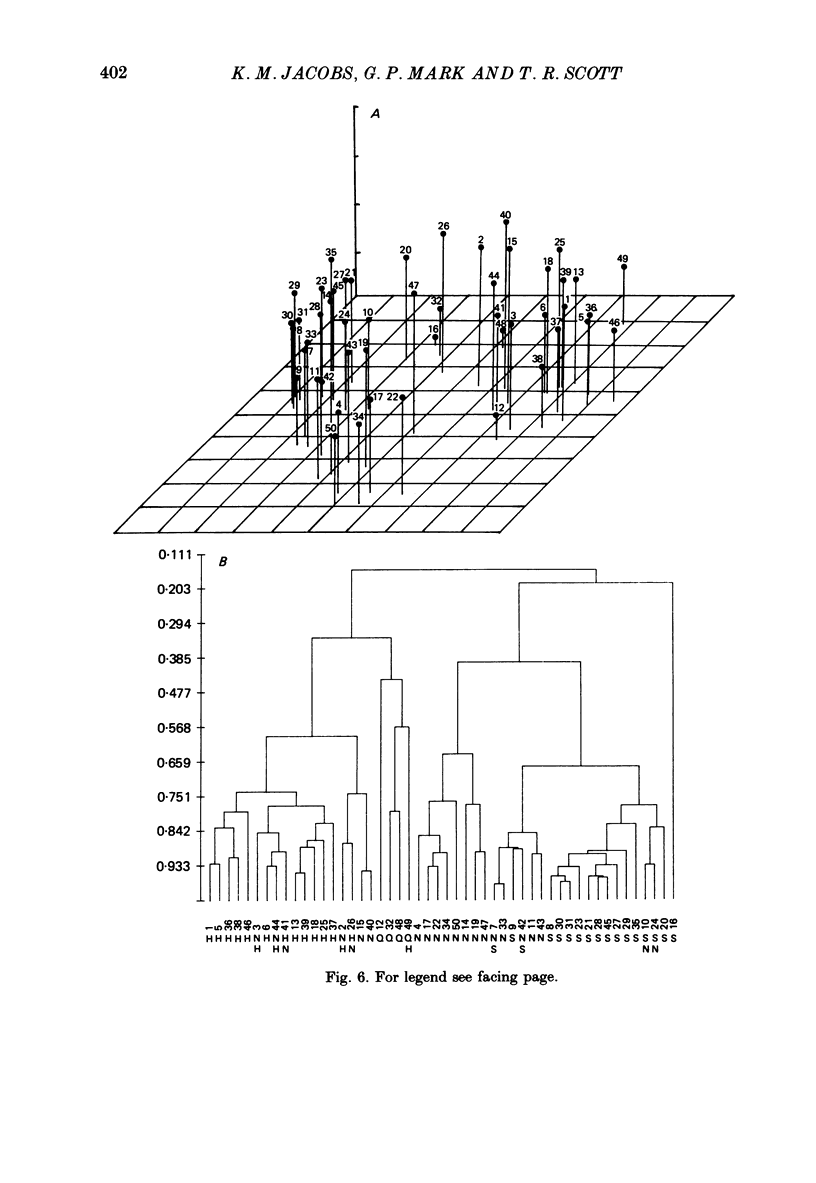


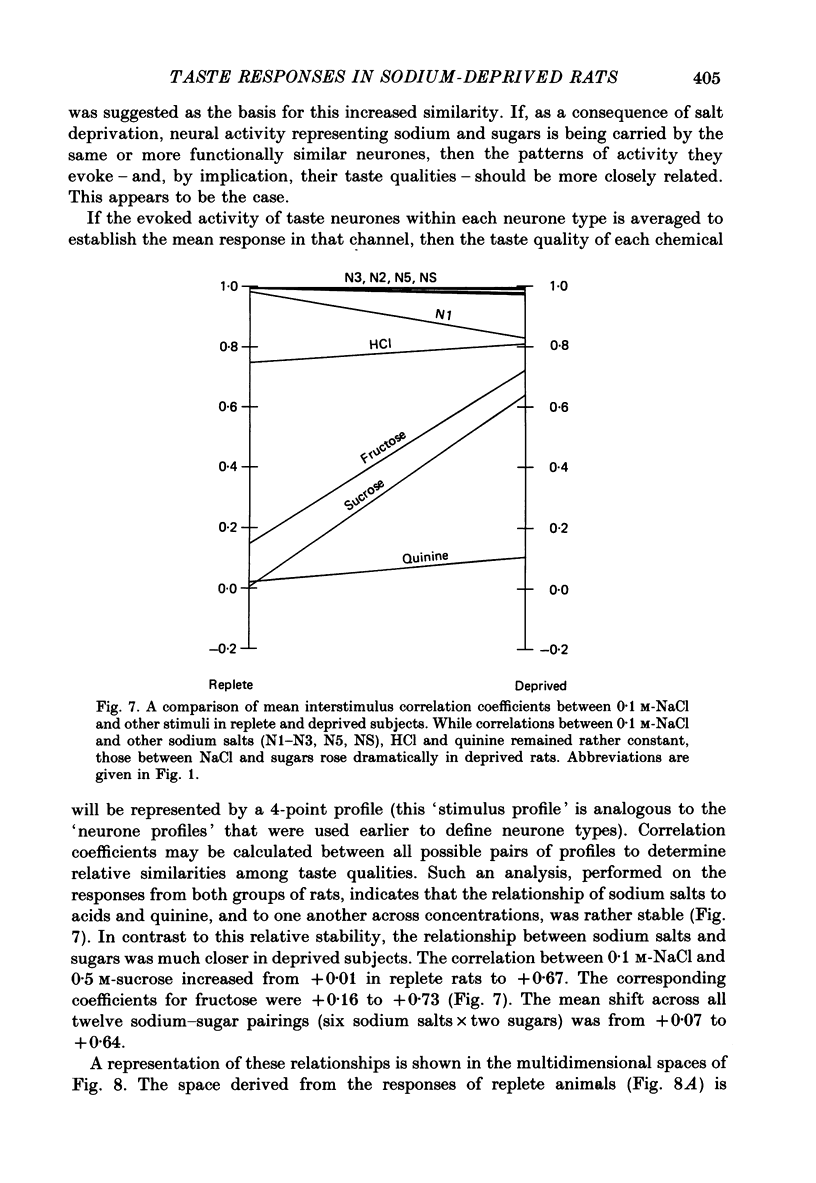
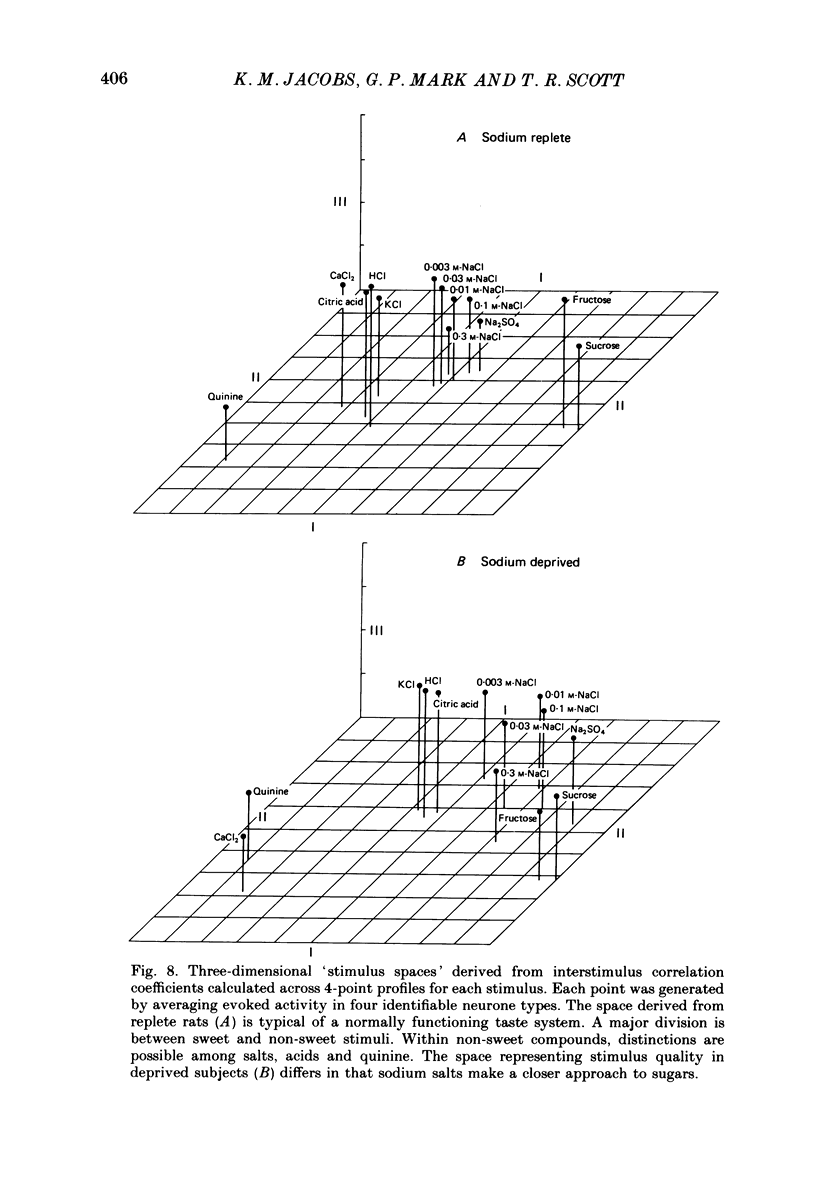




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge K. C., Flynn F. W., Schulkin J., Grill H. J. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984 Aug;98(4):652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- CARR W. J. The effect of adrenalectomy upon the NaC1 taste threshold in rat. J Comp Physiol Psychol. 1952 May;45(4):377–380. doi: 10.1037/h0056378. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J. M., Aghajanian G. K. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978 Mar 1;178(1):1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Chang F. C., Scott T. R. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984 Jul;4(7):1850–1862. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R. J. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977 Feb;121(2):373–378. doi: 10.1016/0006-8993(77)90162-7. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979 May;73(5):569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R. J., Stetson P. W. Changes in salt intake lesions of the area postrema and the nucleus of the solitary tract in rats. Brain Res. 1981 May 4;211(2):355–366. doi: 10.1016/0006-8993(81)90707-1. [DOI] [PubMed] [Google Scholar]
- Cullen J. W., Harriman A. E. Selection of NaCl solutions by sodium-deprived Mongolian gerbils in Richter-type drinking tests. J Psychol. 1973 Mar;83(2D):315–321. doi: 10.1080/00223980.1973.9915619. [DOI] [PubMed] [Google Scholar]
- Doetsch G. S., Erickson R. P. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rate. J Neurophysiol. 1970 Jul;33(4):490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- EPSTEIN A. N., STELLAR E. The control of salt preference in the adrenalectomized rat. J Comp Physiol Psychol. 1955 Jun;48(3):167–172. doi: 10.1037/h0045626. [DOI] [PubMed] [Google Scholar]
- FREGLY M. J., HARPER J. M., Jr, RADFORD E. P., Jr REGULATION OF SODIUM CHLORIDE INTAKE BY RATS. Am J Physiol. 1965 Aug;209:287–292. doi: 10.1152/ajplegacy.1965.209.2.287. [DOI] [PubMed] [Google Scholar]
- FREGLY M. J. Specificity of the sodium chloride appetite of adrenalectomized rats; substitution of lithium chloride for sodium chloride. Am J Physiol. 1958 Dec;195(3):645–653. doi: 10.1152/ajplegacy.1958.195.3.645. [DOI] [PubMed] [Google Scholar]
- Ganchrow J. R., Erickson R. P. Neural correlates of gustatory intensity and quality. J Neurophysiol. 1970 Nov;33(6):768–783. doi: 10.1152/jn.1970.33.6.768. [DOI] [PubMed] [Google Scholar]
- Giza B. K., Scott T. R. Blood glucose selectively affects taste-evoked activity in rat nucleus tractus solitarius. Physiol Behav. 1983 Nov;31(5):643–650. [PubMed] [Google Scholar]
- Giza B. K., Scott T. R. Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars. Am J Physiol. 1987 May;252(5 Pt 2):R994–1002. doi: 10.1152/ajpregu.1987.252.5.R994. [DOI] [PubMed] [Google Scholar]
- Gleen J. F., Erickson R. P. Gastric modulation of gustatory afferent activity. Physiol Behav. 1976 May;16(5):561–568. doi: 10.1016/0031-9384(76)90216-x. [DOI] [PubMed] [Google Scholar]
- Grill H. J., Schulkin J., Flynn F. W. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci. 1986 Aug;100(4):536–543. doi: 10.1037//0735-7044.100.4.536. [DOI] [PubMed] [Google Scholar]
- HARRIMAN A. E. The effect of a preoperative preference for sugar over salt upon compensatory salt selection by adrenalectomized rats. J Nutr. 1955 Oct 10;57(2):271–276. doi: 10.1093/jn/57.2.271. [DOI] [PubMed] [Google Scholar]
- Harriman A. E. Intakes of NaCl by rats in extended two-bottle drinking preference tests. J Psychol. 1967 May;66(1):93–98. doi: 10.1080/00223980.1967.10544884. [DOI] [PubMed] [Google Scholar]
- Mitchell D., Parker L. F., Woods S. C. Cyclophosphamide-induced sodium appetite and hyponatremia in the rat. Pharmacol Biochem Behav. 1974 Sep-Oct;2(5):627–630. doi: 10.1016/0091-3057(74)90031-8. [DOI] [PubMed] [Google Scholar]
- Morrison G. R., Young J. C. Taste control over sodium intake in sodium deficient rats. Physiol Behav. 1972 Jan;8(1):29–32. doi: 10.1016/0031-9384(72)90125-4. [DOI] [PubMed] [Google Scholar]
- NACHMAN M., PFAFFMANN C. GUSTATORY NERVE DISCHARGE IN NORMAL AND SODIUM-DEFICIENT RATS. J Comp Physiol Psychol. 1963 Dec;56:1007–1011. doi: 10.1037/h0044428. [DOI] [PubMed] [Google Scholar]
- NACHMAN M. Taste preferences for sodium salts by adrenalectomized rats. J Comp Physiol Psychol. 1962 Dec;55:1124–1129. doi: 10.1037/h0041348. [DOI] [PubMed] [Google Scholar]
- PFAFFMANN C., BARE J. K. Gustatory nerve discharges in normal and adrenalectomized rats. J Comp Physiol Psychol. 1950 Aug;43(4):320–324. doi: 10.1037/h0059248. [DOI] [PubMed] [Google Scholar]
- Schiffman S. S., Lockhead E., Maes F. W. Amiloride reduces the taste intensity of Na+ and Li+ salts and sweeteners. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6136–6140. doi: 10.1073/pnas.80.19.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. R., Mark G. P. The taste system encodes stimulus toxicity. Brain Res. 1987 Jun 23;414(1):197–203. doi: 10.1016/0006-8993(87)91347-3. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Yaxley S., Sienkiewicz Z. J., Rolls E. T. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986 Sep;56(3):876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Yaxley S., Sienkiewicz Z. J., Rolls E. T. Gustatory responses in the nucleus tractus solitarius of the alert cynomolgus monkey. J Neurophysiol. 1986 Jan;55(1):182–200. doi: 10.1152/jn.1986.55.1.182. [DOI] [PubMed] [Google Scholar]
- Shapiro R. E., Miselis R. R. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985 Apr 15;234(3):344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- Smith D. V., Van Buskirk R. L., Travers J. B., Bieber S. L. Gustatory neuron types in hamster brain stem. J Neurophysiol. 1983 Aug;50(2):522–540. doi: 10.1152/jn.1983.50.2.522. [DOI] [PubMed] [Google Scholar]
- Stricker E. M., Wilson N. E. Salt-seeking behavior in rats following acute sodium deficiency. J Comp Physiol Psychol. 1970 Sep;72(3):416–420. doi: 10.1037/h0029744. [DOI] [PubMed] [Google Scholar]
- WOLF G. EFFECT OF DORSOLATERAL HYPOTHALAMIC LESIONS ON SODIUM APPETITE ELICITED BY DESOXYCORTICOSTERONE AND BY ACUTE HYPONATREMIA. J Comp Physiol Psychol. 1964 Dec;58:396–402. doi: 10.1037/h0048232. [DOI] [PubMed] [Google Scholar]
- WOLF G., STEINBAUM E. A. SODIUM APPETITE ELICTED BY SUBCUTANEOUS FORMALIN: MECHANISM OF ACTION. J Comp Physiol Psychol. 1965 Jun;59:335–339. doi: 10.1037/h0022026. [DOI] [PubMed] [Google Scholar]
- Wolf G., Handal P. J. Aldosterone-induced sodium appetite: dose-response and specificity. Endocrinology. 1966 Jun;78(6):1120–1124. doi: 10.1210/endo-78-6-1120. [DOI] [PubMed] [Google Scholar]
- Wolf G., Quartermain D. Sodium chloride intake of desoxycorticosterone-treated and of sodium-deficient rats as a function of saline concentration. J Comp Physiol Psychol. 1966 Apr;61(2):288–291. doi: 10.1037/h0023132. [DOI] [PubMed] [Google Scholar]
- Woolston D. C., Erickson R. P. Concept of neuron types in gustation in the rat. J Neurophysiol. 1979 Sep;42(5):1390–1409. doi: 10.1152/jn.1979.42.5.1390. [DOI] [PubMed] [Google Scholar]
- van der Kooy D., Koda L. Y. Organization of the projections of a circumventricular organ: the area postrema in the rat. J Comp Neurol. 1983 Sep 20;219(3):328–338. doi: 10.1002/cne.902190307. [DOI] [PubMed] [Google Scholar]


