Abstract
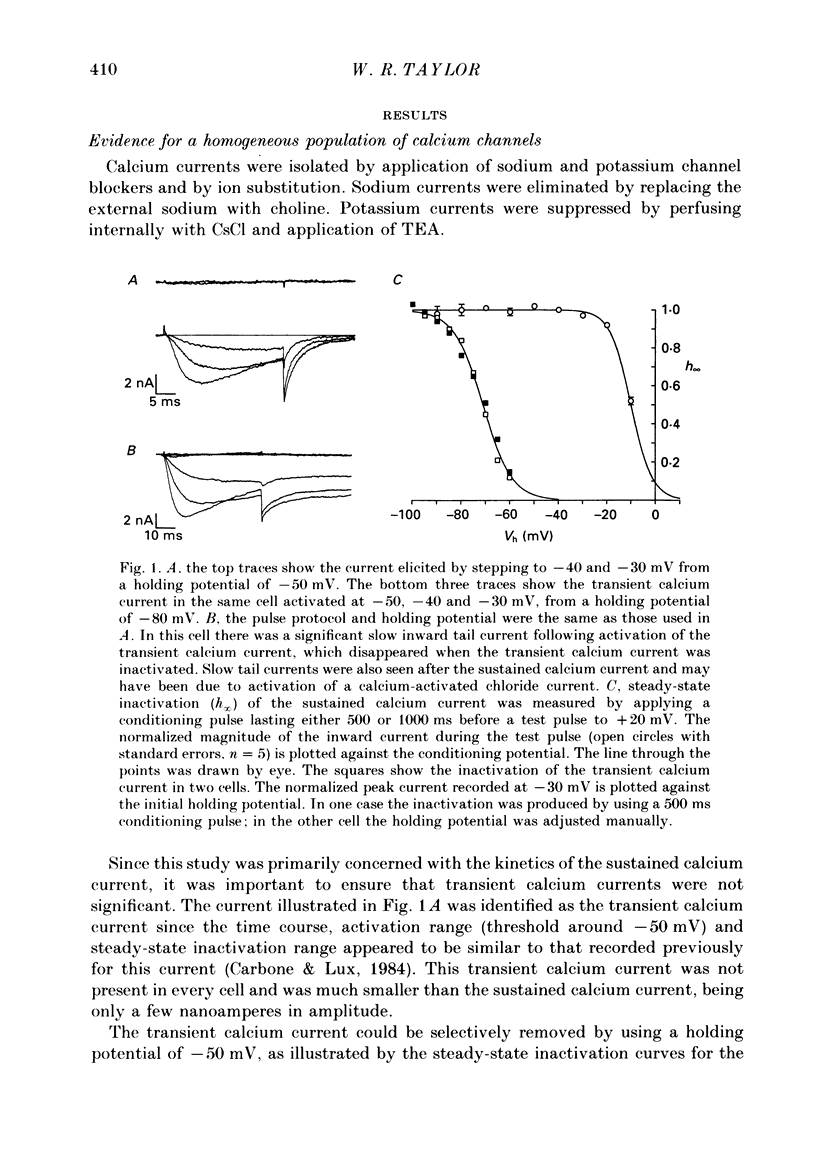
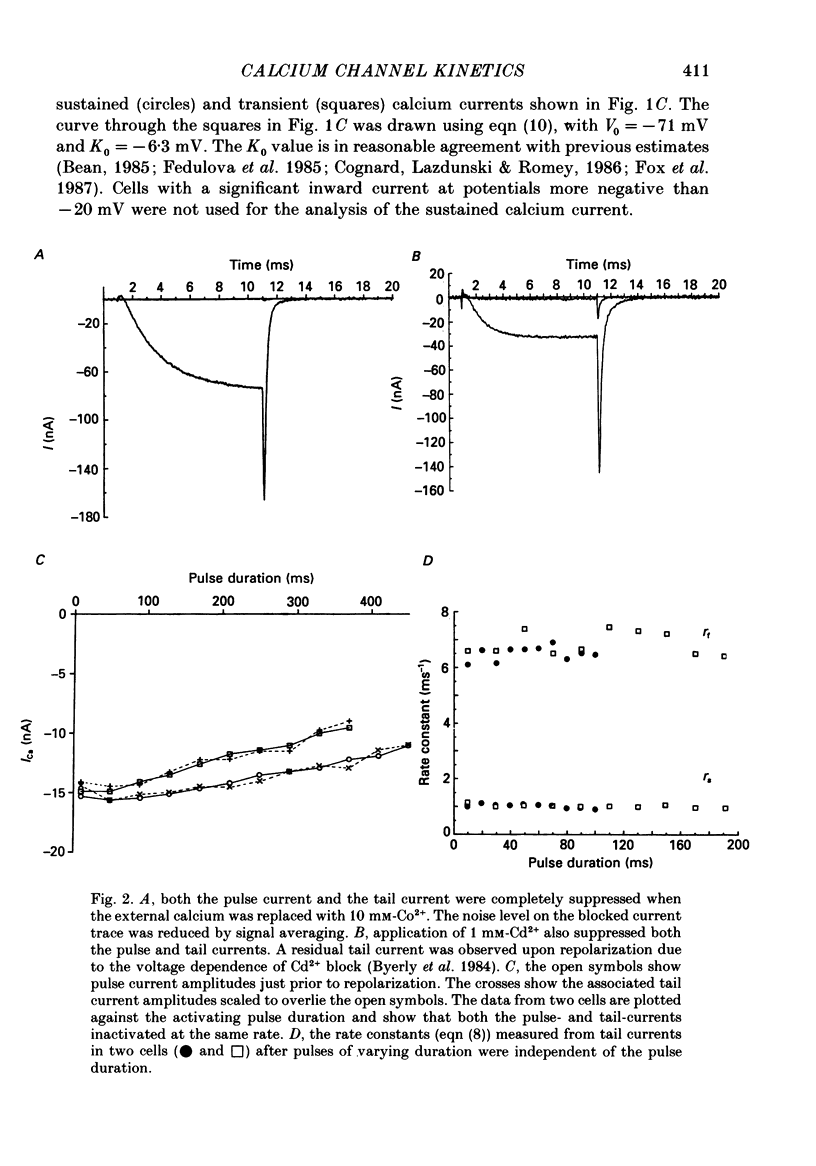
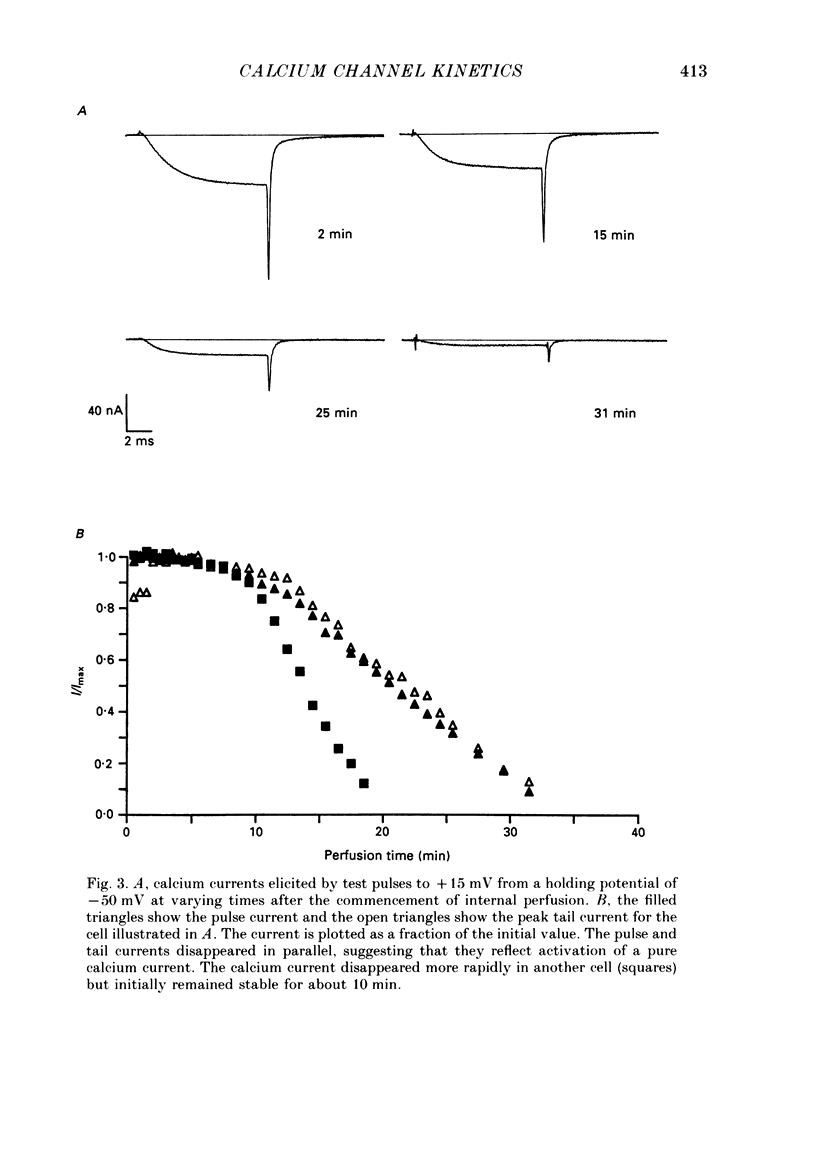
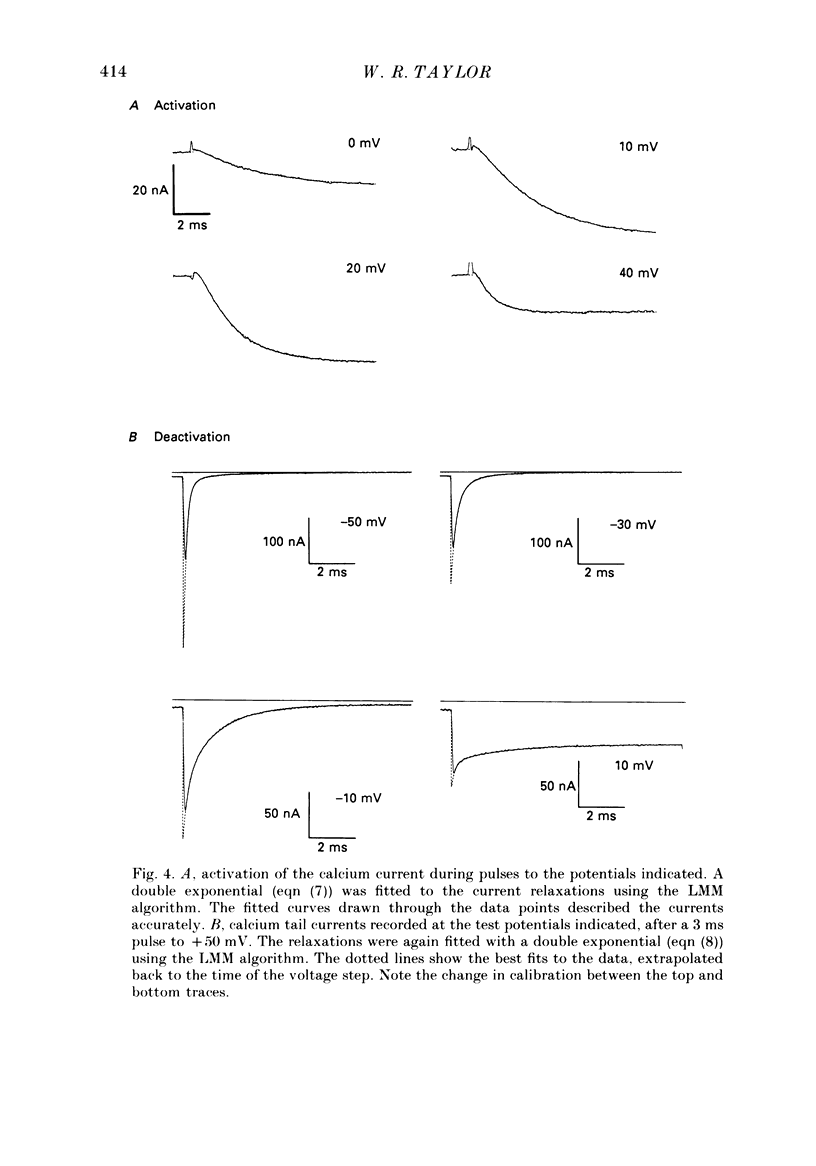
1. The kinetics of the sustained calcium current were examined in cat dorsal root ganglion (DRG) neurones, using a two-suction-electrode voltage clamp. It was shown that this current could be examined with minimal contamination from other ionic currents. Experiments were performed at 20 degrees C, with a concentration of 10 mM-calcium externally. 2. The transient calcium current was eliminated by using a holding potential of -50 mV. The sustained calcium current showed no evidence of steady-state inactivation at potentials between -90 and -30 mV. 3. The activation and deactivation time course of the calcium current was described by a double-exponential function. The activation process was examined without interference from significant inactivation under the conditions used. 4. The steady-state activation of the calcium channels was approximated by a two-step activation process. Both reactions were voltage sensitive, the first having an equivalent valency of 3.4 +/- 0.1 electronic charges (e-), and the second with an equivalent valency of 1.0 +/- 0.2 e-. On average, half-maximal channel activation occurred at +10 mV. 5. The fast and the slow time constants of the exponential relaxations differed by a factor of 4-10 and both showed significant voltage dependence. Both the fast and slow time constants were greatest at potentials where approximately half the available channels were activated in the steady state. The slow time constant measured from activation and deactivation appeared to be independent of the starting potential. 6. The fractional amplitude of the slow exponential component of the tail currents was 0.09 +/- 0.01 at -70 mV and increased steadily at more positive potentials passing through a clear maximum of 0.59 +/- 0.03 at -10 mV. 7. Reducing the temperature decreased the magnitude of the peak inward current, with an apparent activation energy (Ea) of 67 kJ/mol. The slow time constants measured from activation and deactivation were also reduced at lower temperatures. The slow activation time constant had a higher temperature sensitivity (Ea = 85 kJ/mol) than the slow tail current time constant (Ea = 29 kJ/mol). The fast tail current time constant was reduced at lower temperatures with an apparent Ea of 79 kJ/mol. 8. Application of 10 microM-(+/-)Bay K 8644 prolonged the tail currents and shortened the activation time constant. The calcium current was activated at more negative potentials and the slope of the steady-state activation curve was reduced.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





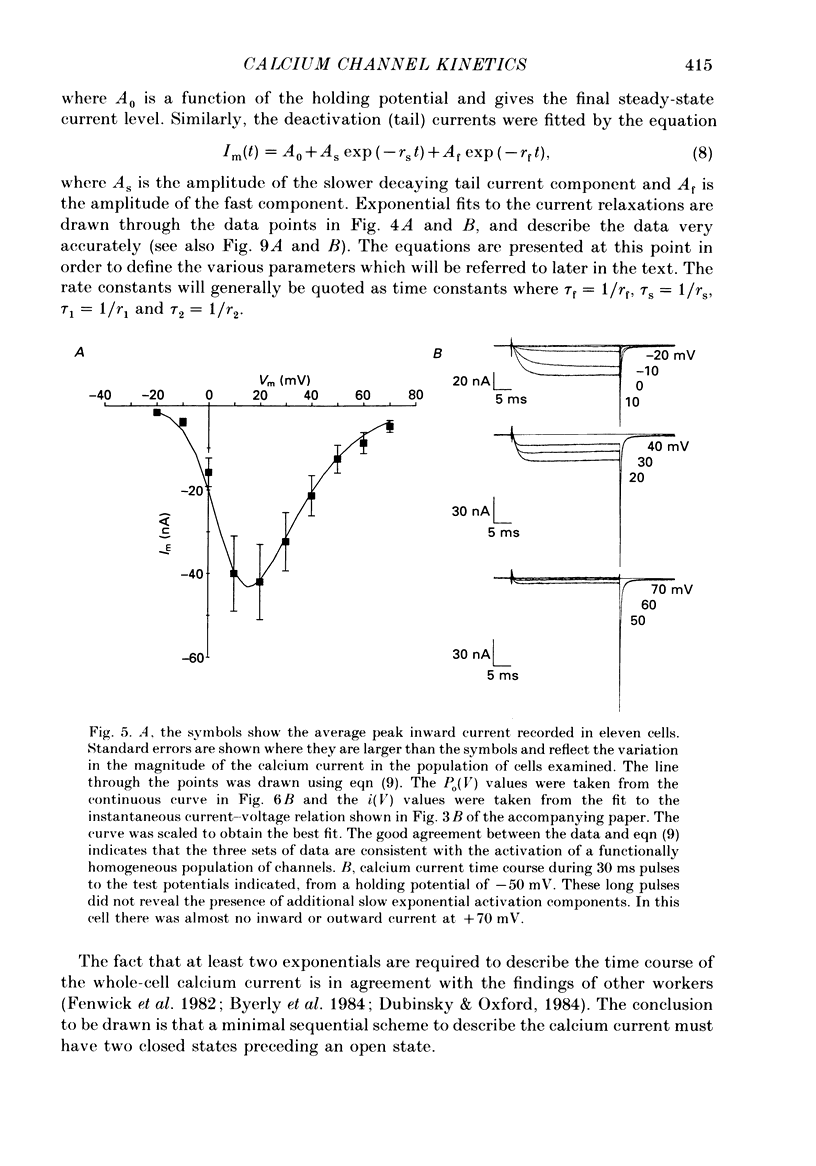
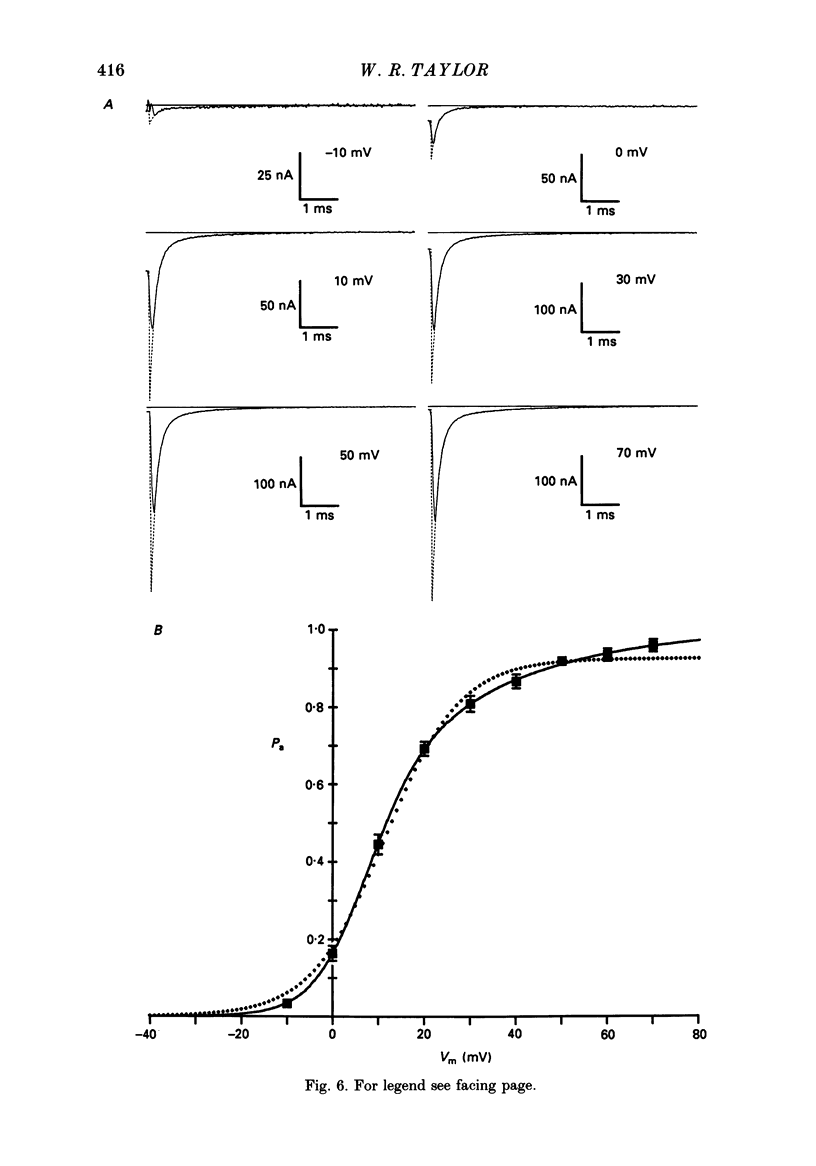


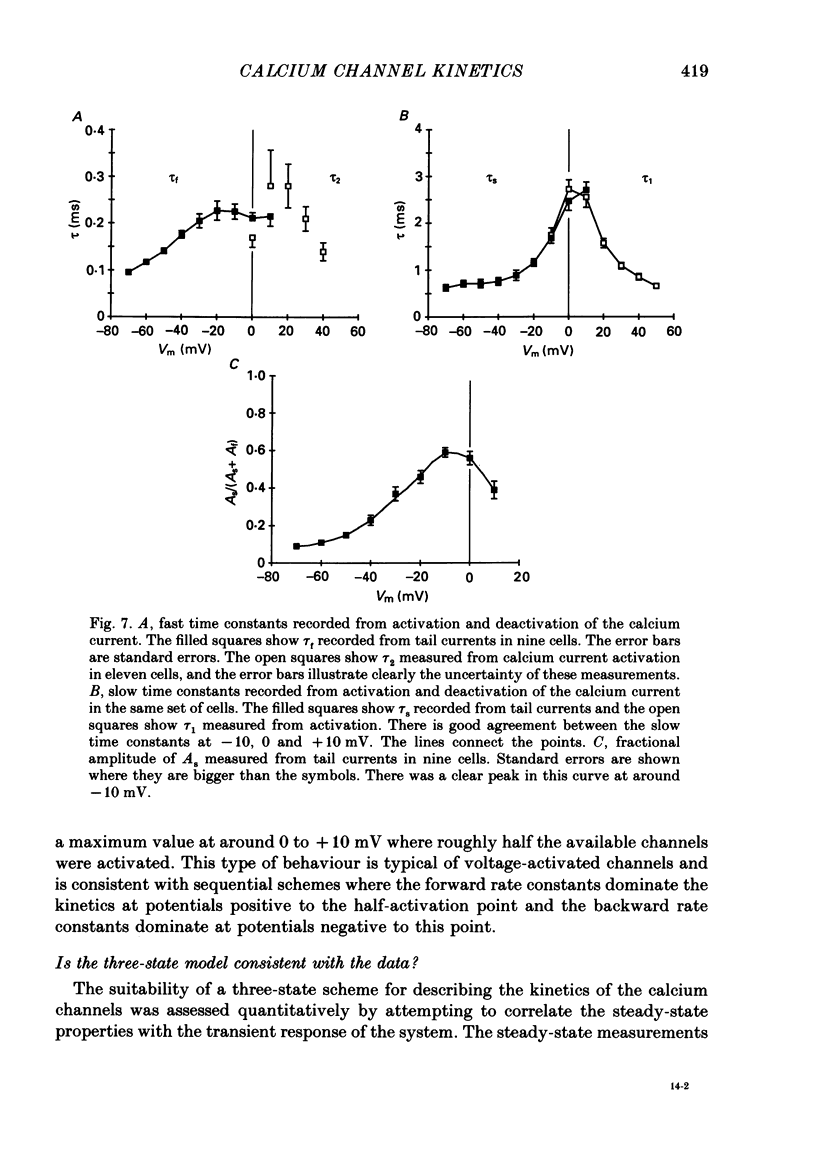

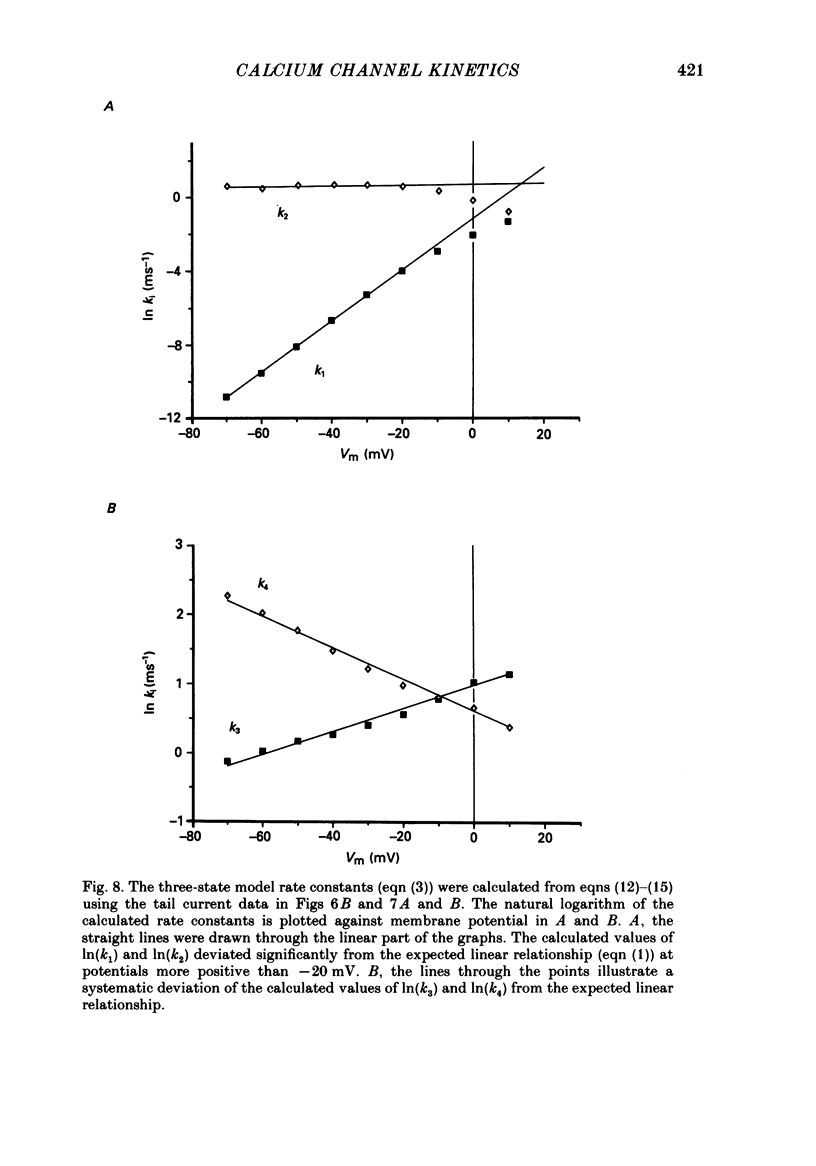



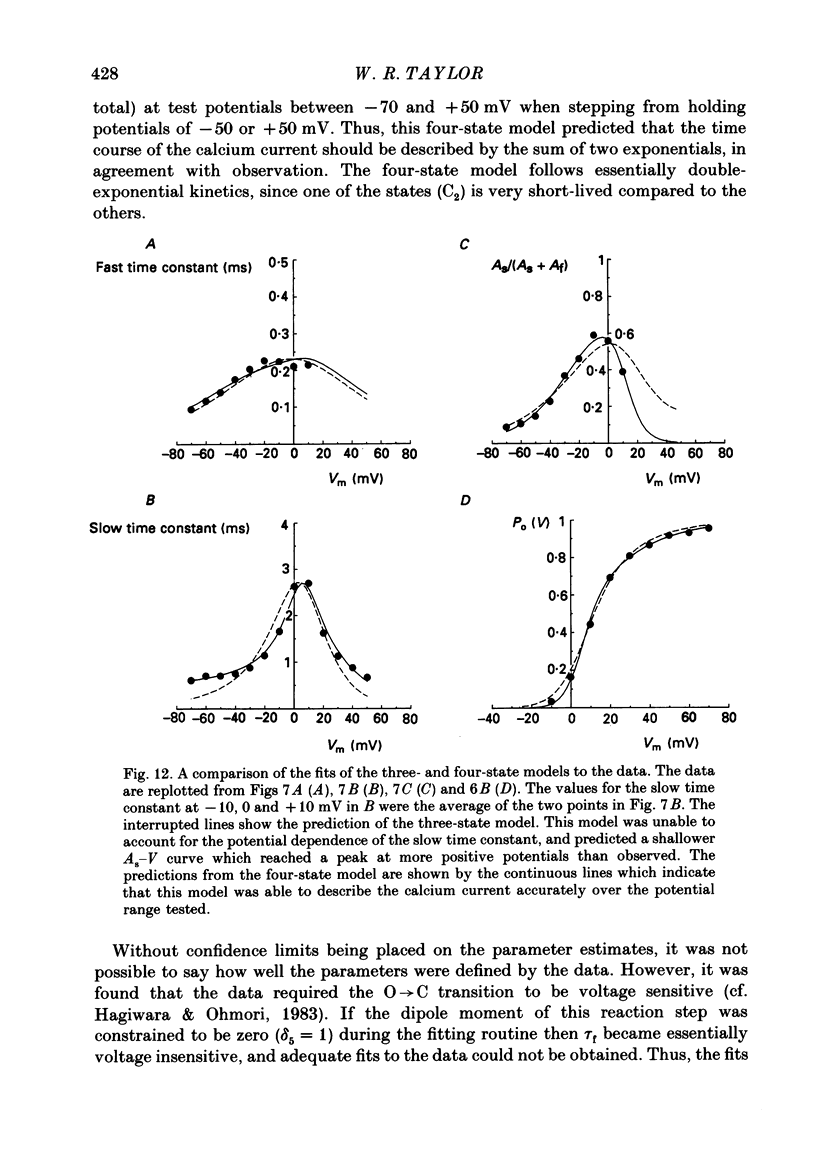




Selected References
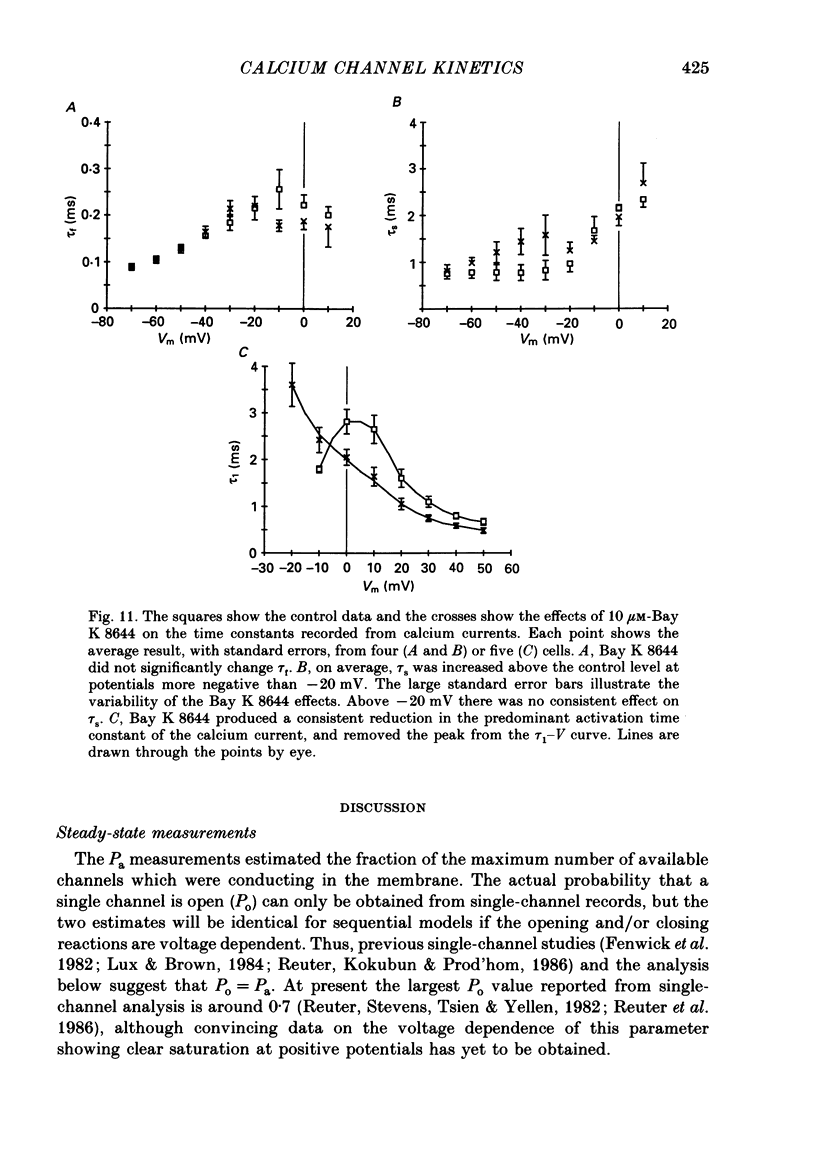
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
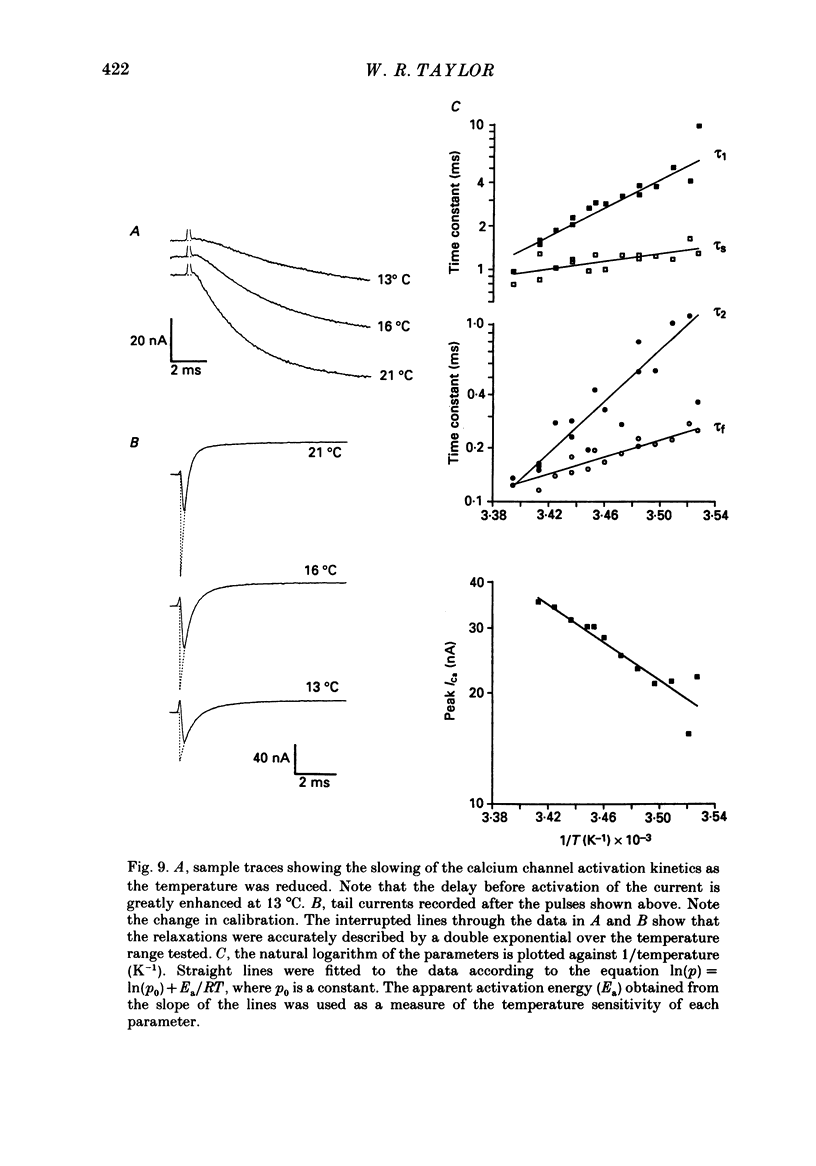
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
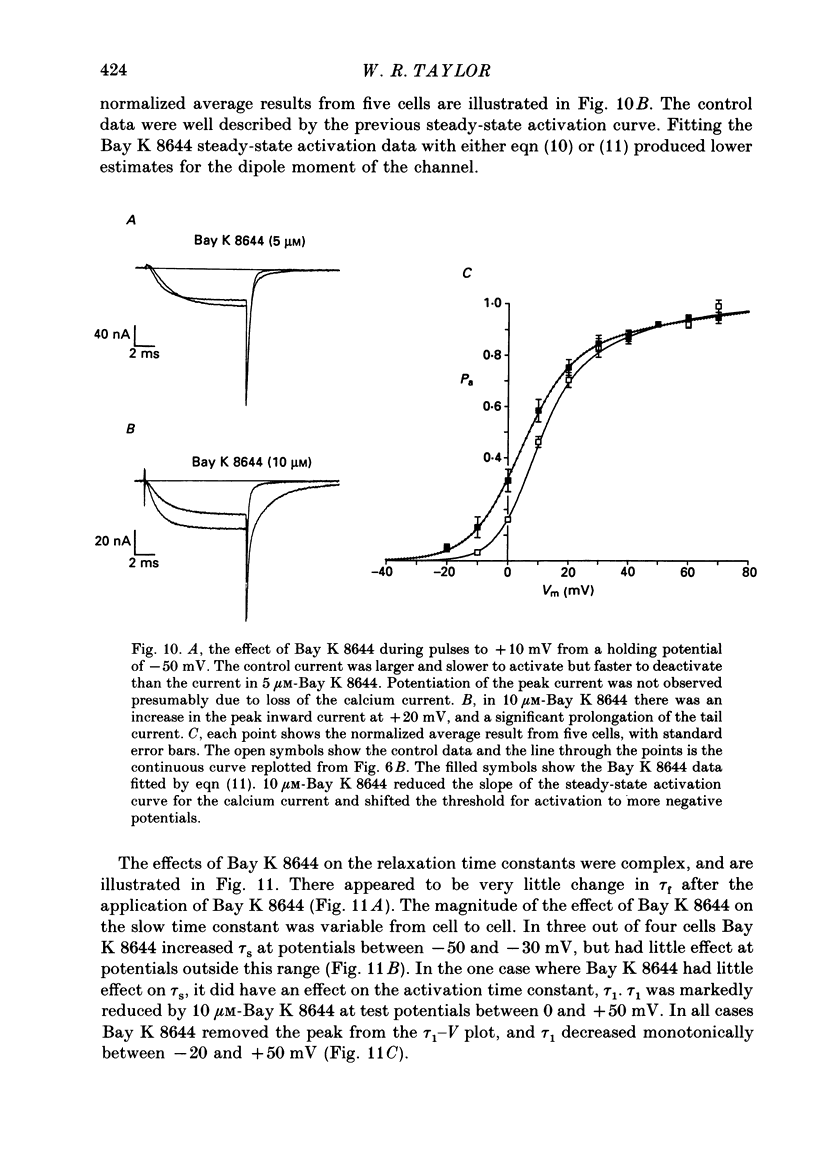
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lux H. D., Wilson D. L. Activation and inactivation of single calcium channels in snail neurons. J Gen Physiol. 1984 May;83(5):751–769. doi: 10.1085/jgp.83.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Tsuda Y., Wilson D. L. A description of activation and conduction in calcium channels based on tail and turn-on current measurements in the snail. J Physiol. 1983 Nov;344:549–583. doi: 10.1113/jphysiol.1983.sp014956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Chase P. B., Stimers J. R. Calcium current activation kinetics in neurones of the snail Lymnaea stagnalis. J Physiol. 1984 Mar;348:187–207. doi: 10.1113/jphysiol.1984.sp015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cognard C., Lazdunski M., Romey G. Different types of Ca2+ channels in mammalian skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1986 Jan;83(2):517–521. doi: 10.1073/pnas.83.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H. Electrically gated ionic channels in lipid bilayers. Q Rev Biophys. 1977 Feb;10(1):1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franckowiak G., Bechem M., Schramm M., Thomas G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol. 1985 Aug 15;114(2):223–226. doi: 10.1016/0014-2999(85)90631-4. [DOI] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of single calcium channel currents in rat clonal pituitary cells. J Physiol. 1983 Mar;336:649–661. doi: 10.1113/jphysiol.1983.sp014603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hof R. P., Rüegg U. T., Hof A., Vogel A. Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol. 1985 Jul-Aug;7(4):689–693. doi: 10.1097/00005344-198507000-00012. [DOI] [PubMed] [Google Scholar]
- Junge D., Miller J. Different spike mechanisms in axon and soma of molluscan neurone. Nature. 1974 Nov 8;252(5479):155–156. doi: 10.1038/252155a0. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Calcium current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol. 1987 Nov;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Doroshenko P. A. Calcium currents in snail neurones. I. Identification of calcium current. Pflugers Arch. 1974 Apr 11;348(2):83–93. doi: 10.1007/BF00586471. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Patch and whole cell calcium currents recorded simultaneously in snail neurons. J Gen Physiol. 1984 May;83(5):727–750. doi: 10.1085/jgp.83.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Kokubun S., Prod'hom B. Properties and modulation of cardiac calcium channels. J Exp Biol. 1986 Sep;124:191–201. doi: 10.1242/jeb.124.1.191. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Interactions between intrinsic membrane protein and electric field. An approach to studying nerve excitability. Biophys J. 1978 May;22(2):295–306. doi: 10.1016/S0006-3495(78)85490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


