Abstract
OBJECTIVE: To examine the effects of cholestatic jaundice on gut barrier function. SUMMARY BACKGROUND DATA: Gut barrier failure occurs in animal models of jaundice. In humans, the presence of endotoxemia indirectly implicates failure of this host defense, but this has not previously been investigated in jaundiced patients. METHODS: Twenty-seven patients with extrahepatic obstructive jaundice and 27 nonicteric subjects were studied. Intestinal permeability was measured using the lactulose-mannitol test. Small intestinal morphology and the presence of mucosal immunologic activation were examined in endoscopic biopsies of the second part of the duodenum. Systemic antiendotoxin core IgG antibodies and serum interleukin-6 and C-reactive protein were also quantified. Intestinal permeability was remeasured in 9 patients 5 weeks after internal biliary drainage. RESULTS: The median lactulose-mannitol ratio was significantly increased in the jaundiced patients. This was accompanied by upregulation of HLA-DR expression on enterocytes and gut-associated lymphoid tissue, suggesting immune activation. A significant increase in the acute phase response and circulating antiendotoxin core antibodies was also observed in the jaundiced patients. After internal biliary drainage, intestinal permeability returned toward normal levels. CONCLUSIONS: A reversible impairment in gut barrier function occurs in patients with cholestatic jaundice. Increased intestinal permeability is associated with local immune cell and enterocyte activation. In view of the role of gut defenses in the modern paradigm of sepsis, these data may directly identify an important underlying mechanism contributing to the high risk of sepsis in jaundiced patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. P., Detsky A. S., Wesson D. E., Wolman S. L., Stewart S., Whitewell J., Langer B., Jeejeebhoy K. N. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982 Apr 22;306(16):969–972. doi: 10.1056/NEJM198204223061606. [DOI] [PubMed] [Google Scholar]
- Barclay G. R. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Huitfeldt H. S., Krajci P., Kvale D., Scott H., Thrane P. S. Epithelial expression of HLA, secretory component (poly-Ig receptor), and adhesion molecules in the human alimentary tract. Ann N Y Acad Sci. 1992;664:157–179. doi: 10.1111/j.1749-6632.1992.tb39758.x. [DOI] [PubMed] [Google Scholar]
- Budillon G., Parrilli G., Pacella M., Cuomo R., Menzies I. S. Investigation of intestine and liver function in cirrhosis using combined sugar oral loads. J Hepatol. 1985;1(5):513–524. doi: 10.1016/s0168-8278(85)80749-2. [DOI] [PubMed] [Google Scholar]
- Cahill C. J. Prevention of postoperative renal failure in patients with obstructive jaundice--the role of bile salts. Br J Surg. 1983 Oct;70(10):590–595. doi: 10.1002/bjs.1800701008. [DOI] [PubMed] [Google Scholar]
- Clements W. D., Halliday M. I., McCaigue M. D., Barclay R. G., Rowlands B. J. Effects of extrahepatic obstructive jaundice on Kupffer cell clearance capacity. Arch Surg. 1993 Feb;128(2):200–205. doi: 10.1001/archsurg.1993.01420140077012. [DOI] [PubMed] [Google Scholar]
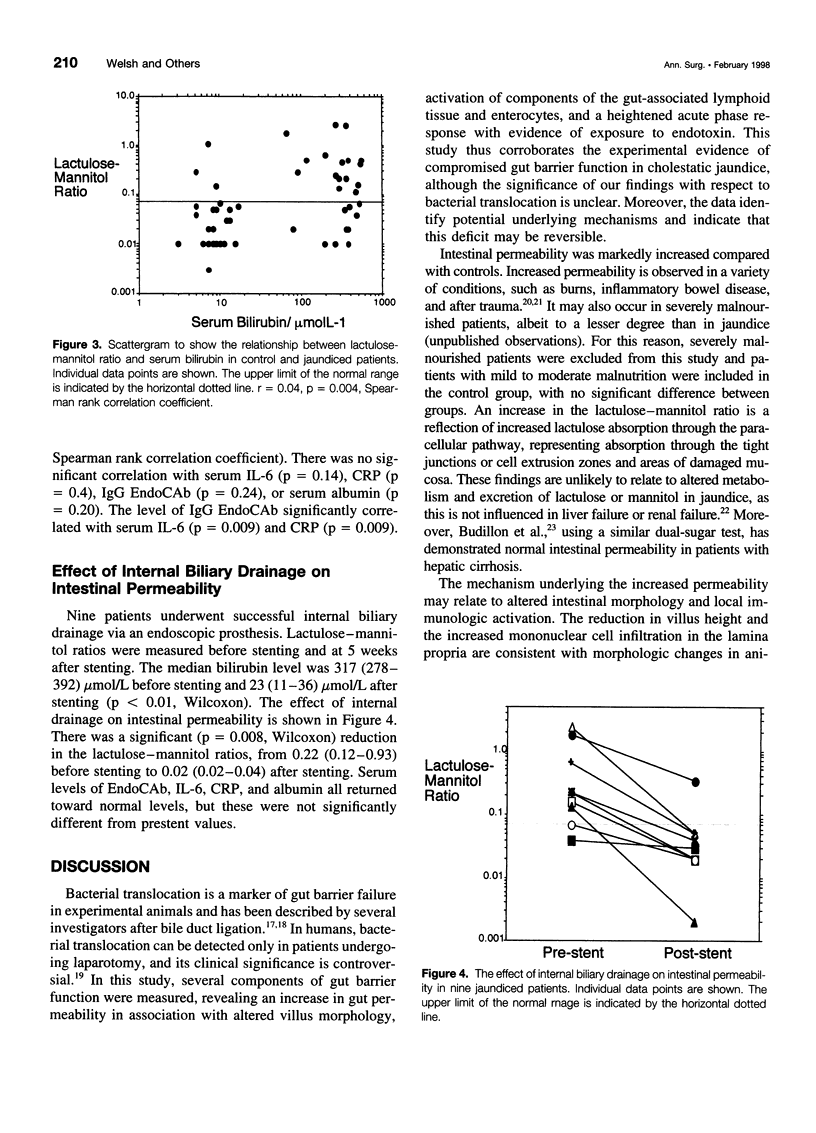
- Clements W. D., McCaigue M., Erwin P., Halliday I., Rowlands B. J. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut. 1996 Jun;38(6):925–931. doi: 10.1136/gut.38.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Hamilton I., Rothwell J., Axon A. T. Cellobiose/mannitol test: physiological properties of probe molecules and influence of extraneous factors. Clin Chim Acta. 1985 May 15;148(1):53–62. doi: 10.1016/0009-8981(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Berg R., Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987 Feb;122(2):185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINE J., FRANK E. D., RAVIN H. A., RUTENBERG S. H., SCHWEINBURG F. B. The bacterial factor in traumatic shock. N Engl J Med. 1959 Jan 29;260(5):214–220. doi: 10.1056/NEJM195901292600505. [DOI] [PubMed] [Google Scholar]
- Goris R. J., te Boekhorst T. P., Nuytinck J. K., Gimbrère J. S. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985 Oct;120(10):1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- Gouma D. J., Coelho J. C., Fisher J. D., Schlegel J. F., Li Y. F., Moody F. G. Endotoxemia after relief of biliary obstruction by internal and external drainage in rats. Am J Surg. 1986 Apr;151(4):476–479. doi: 10.1016/0002-9610(86)90107-8. [DOI] [PubMed] [Google Scholar]
- Gouma D. J., Coelho J. C., Schlegel J. F., Li Y. F., Moody F. G. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch Surg. 1987 Jun;122(6):731–734. doi: 10.1001/archsurg.1987.01400180113022. [DOI] [PubMed] [Google Scholar]
- Hatfield A. R., Tobias R., Terblanche J., Girdwood A. H., Fataar S., Harries-Jones R., Kernoff L., Marks I. N. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982 Oct 23;2(8304):896–899. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- Holman J. M., Jr, Rikkers L. F., Moody F. G. Sepsis in the management of complicated biliary disorders. Am J Surg. 1979 Dec;138(6):809–813. doi: 10.1016/0002-9610(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Jiang W. G., Puntis M. C., Hallett M. B. Neutrophil priming by cytokines in patients with obstructive jaundice. HPB Surg. 1994;7(4):281–289. doi: 10.1155/1994/74202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juby L. D., Rothwell J., Axon A. T. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989 Jan;96(1):79–85. doi: 10.1016/0016-5085(89)90767-1. [DOI] [PubMed] [Google Scholar]
- Lygidakis N. J., van der Heyde M. N., Lubbers M. J. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987 Nov-Dec;153(11-12):665–668. [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A., Benjamin I. S., Hodgson H. J., Bowley N. B., Allison D. J., Blumgart L. H. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984 May;71(5):371–375. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- Menzies I. S. Quantitative estimation of sugars in blood and urine by paper chromatography using direct densitometry. J Chromatogr. 1973 Jun 27;81(1):109–127. doi: 10.1016/s0021-9673(01)82322-0. [DOI] [PubMed] [Google Scholar]
- Pain J. A., Bailey M. E. Experimental and clinical study of lactulose in obstructive jaundice. Br J Surg. 1986 Oct;73(10):775–778. doi: 10.1002/bjs.1800731003. [DOI] [PubMed] [Google Scholar]
- Pape H. C., Dwenger A., Regel G., Auf'm'Kolck M., Gollub F., Wisner D., Sturm J. A., Tscherne H. Increased gut permeability after multiple trauma. Br J Surg. 1994 Jun;81(6):850–852. doi: 10.1002/bjs.1800810619. [DOI] [PubMed] [Google Scholar]
- Pitt H. A., Gomes A. S., Lois J. F., Mann L. L., Deutsch L. S., Longmire W. P., Jr Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985 May;201(5):545–553. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. V. Gut barrier function in the surgical patient. Br J Surg. 1996 Dec;83(12):1668–1669. doi: 10.1002/bjs.1800831204. [DOI] [PubMed] [Google Scholar]
- Reynolds J. V., Murchan P., Redmond H. P., Watson R. W., Leonard N., Hill A., Clarke P., Marks P., Keane F. B., Tanner W. A. Failure of macrophage activation in experimental obstructive jaundice: association with bacterial translocation. Br J Surg. 1995 Apr;82(4):534–538. doi: 10.1002/bjs.1800820432. [DOI] [PubMed] [Google Scholar]
- Saitoh N., Hiraoka T., Uchino R., Miyauchi Y. Endotoxemia and intestinal mucosal dysfunction after the relief of obstructive jaundice by internal and external drainage in rats. Eur Surg Res. 1995;27(1):11–18. doi: 10.1159/000129367. [DOI] [PubMed] [Google Scholar]
- Scott B. B., Barclay G. R. Endotoxin-polymyxin complexes in an improved enzyme-linked immunosorbent assay for IgG antibodies in blood donor sera to gram-negative endotoxin core glycolipids. Vox Sang. 1987;52(4):272–280. doi: 10.1111/j.1423-0410.1987.tb04893.x. [DOI] [PubMed] [Google Scholar]
- Slocum M. M., Sittig K. M., Specian R. D., Deitch E. A. Absence of intestinal bile promotes bacterial translocation. Am Surg. 1992 May;58(5):305–310. [PubMed] [Google Scholar]
- Smith R. C., Pooley M., George C. R., Faithful G. R. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985 Jun;97(6):641–648. [PubMed] [Google Scholar]
- Sturgess R. P., Hooper L. B., Spencer J., Hung C. H., Nelufer J. M., Ciclitira P. J. Effects of interferon-gamma and tumour necrosis factor-alpha on epithelial HLA class-II expression on jejunal mucosal biopsy specimens cultured in vitro. Scand J Gastroenterol. 1992 Nov;27(11):907–911. doi: 10.3109/00365529209000161. [DOI] [PubMed] [Google Scholar]
- Trede M., Schwall G. The complications of pancreatectomy. Ann Surg. 1988 Jan;207(1):39–47. doi: 10.1097/00000658-198801000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane D. W., Redlich P., Weber T., Leapman S., Siddiqui A. R., Grosfeld J. L. Impaired immune function in obstructive jaundice. J Surg Res. 1988 Sep;45(3):287–293. doi: 10.1016/0022-4804(88)90077-7. [DOI] [PubMed] [Google Scholar]
- Wardle E. N., Wright N. A. Endotoxin and acute renal failure associated with obstructive jaundice. Br Med J. 1970 Nov 21;4(5733):472–474. doi: 10.1136/bmj.4.5733.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., Jechorek R. P., Erlandsen S. L. Inhibitory effect of bile on bacterial invasion of enterocytes: possible mechanism for increased translocation associated with obstructive jaundice. Crit Care Med. 1995 Feb;23(2):301–307. doi: 10.1097/00003246-199502000-00016. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Smith R. J., O'Dwyer S. T., Demling R. H., Wilmore D. W. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988 Nov;123(11):1313–1319. doi: 10.1001/archsurg.1988.01400350027003. [DOI] [PubMed] [Google Scholar]



