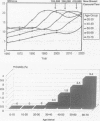
Abstract
OBJECTIVE: To define preliminary guidelines for the use of lymphatic mapping techniques in patients with breast cancer. SUMMARY BACKGROUND DATA: Lymphatic mapping techniques have the potential of changing the standard of surgical care of patients with breast cancer. METHODS: Four hundred sixty-six consecutive patients with newly diagnosed breast cancer underwent a prospective trial of intraoperative lymphatic mapping using a combination of vital blue dye and filtered technetium-labeled sulfur colloid. A sentinel lymph node (SLN) was defined as a blue node and/or a hot node with a 10:1 ex vivo gamma probe ratio of SLN to non-SLN. All SLNs were bivalved, step-sectioned, and examined with routine hematoxylin and eosin (H&E) stains and immunohistochemical stains for cytokeratin. A cytokeratin-positive SLN was defined as any SLN with a defined cluster of positive-staining cells that could be confirmed histologically on H&E sections. RESULTS: Fine-needle aspiration (FNA) or stereotactic core biopsy was used to diagnose 195 of the 422 patients (46.2%) with breast cancer; 227 of 422 patients (53.8%) were diagnosed by excisional biopsy. The SLN was successfully identified in 440 of 466 patients (94.4%). Failure to identify an SLN to the axilla intraoperatively occurred in 26 of 466 patients (5.6%). In all patients who failed lymphatic mappings, a complete axillary dissection was performed, and metastatic disease was documented in 4 of 26 (15.4%) of these patients. Of the 26 patients who failed lymphatic mapping, 11 of 227 (4.8%) were diagnosed by excisional biopsy and 15 of 195 (7.7%) were diagnosed by FNA or stereotactic core biopsy. Of interest, there was only one skip metastasis (defined as a negative SLN with higher nodes in the chain being positive) in a patient with prior excisional biopsy. A mean of 1.92 SLNs were harvested per patient. Twenty percent of the SLNs removed were positive for metastatic disease in 105 of 440 (23.8%) of the patients. Descriptive information on 844 SLNs was evaluated: 339 of 844 (40.2%) were hot, 272 of 844 (32.2%) were blue, and 233 of 844 (27.6%) were both hot and blue. At least one positive SLN was found in 4 of 87 patients (4.6%) with noninvasive (ductal carcinoma in situ) tumors. A greater incidence of positive SLNs was found in patients who had invasive tumors of increasing size: 18 of 112 patients (16%) with tumor size between 0.1 mm and 1 cm had positive SLNs. However, a significantly greater percentage of patients (43 of 131 [32.8%] with tumor size between 1 and 2 cm and 31 of 76 [40.8%] with tumor size between 2 and 5 cm) had positive SLNs. The highest incidence of positive SLNs was seen with patients of tumor size greater than 5 cm; in this group, 9 of 12 (75%) had a positive SLN (p < 0.001). CONCLUSIONS: This study demonstrates that accurate SLN identification was obtained when all blue and hot lymph nodes were harvested as SLNs. Therefore, lymphatic mapping and SLN biopsy is most effective when a combination of vital blue dye and radiolabeled sulfur colloid is used. Furthermore, these data demonstrate that patients with ductal carcinoma in situ or small tumors exhibit a low but significant incidence of metastatic disease to the axillary lymph nodes and may benefit most from selective lymphadenectomy, avoiding the unnecessary complications of a complete axillary lymph node dissection.
Full text
PDF
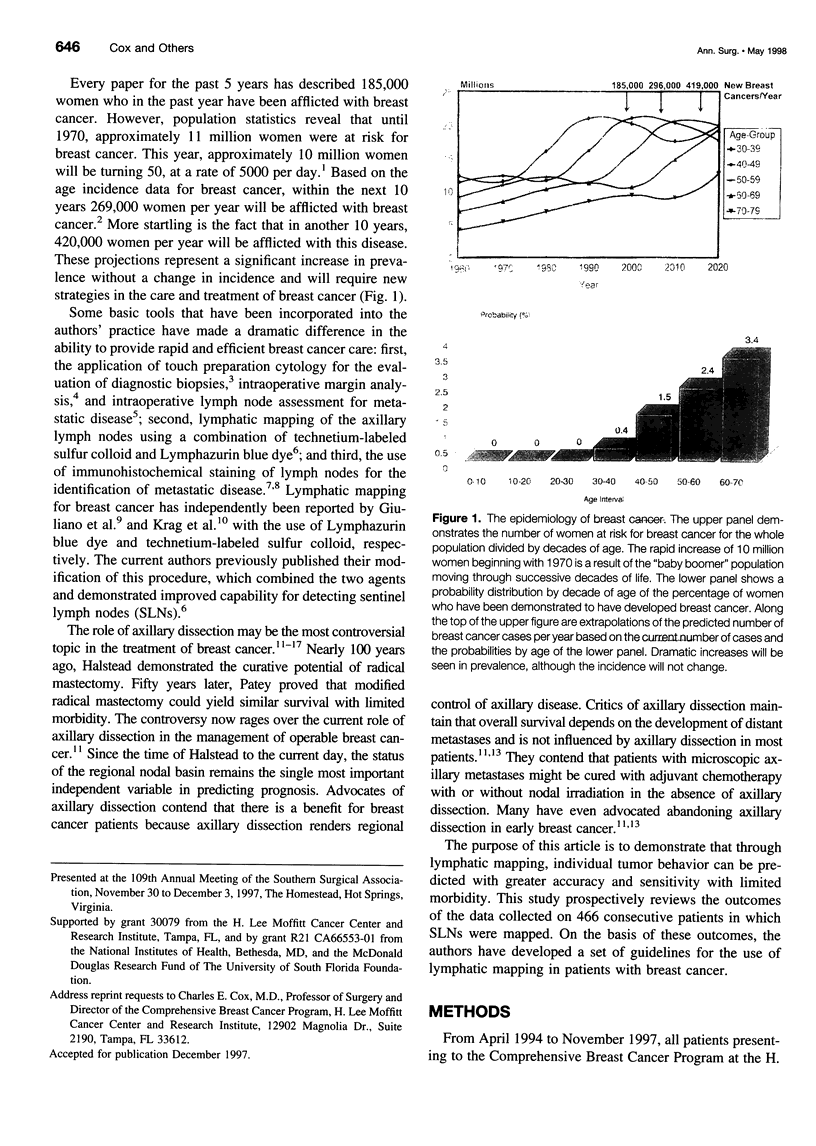

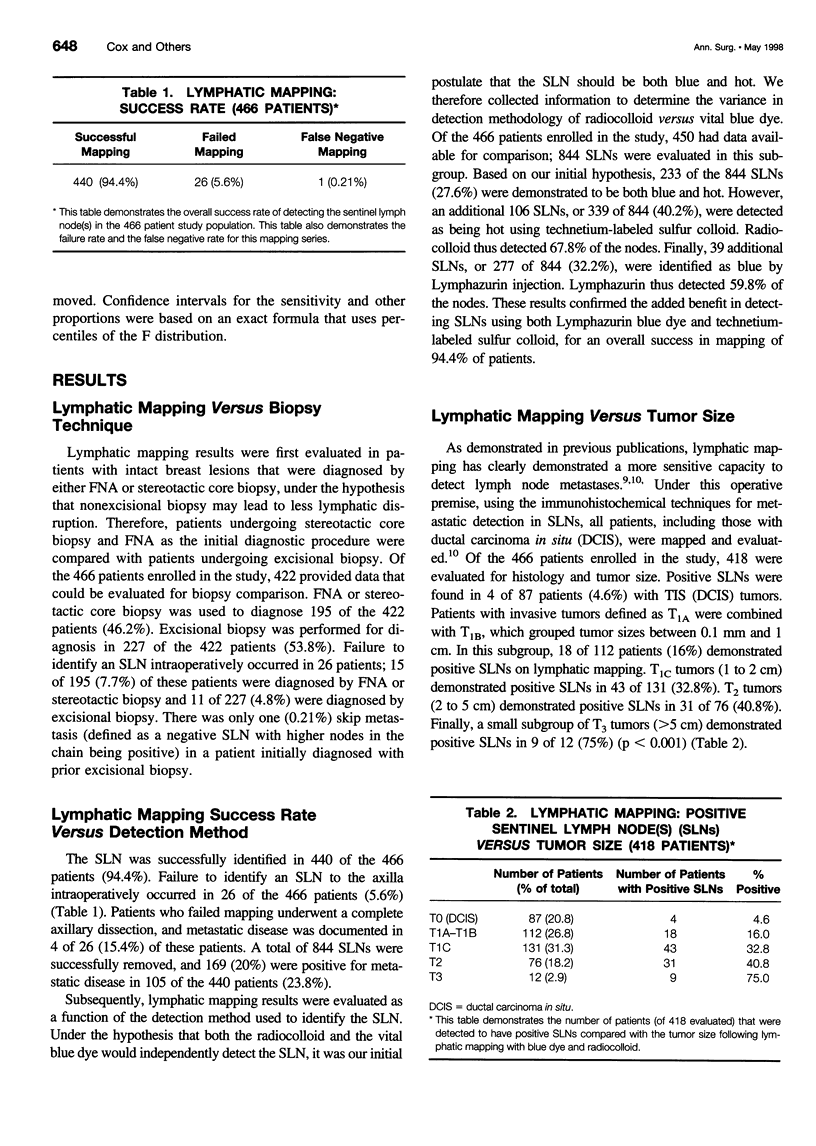



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini J. J., Lyman G. H., Cox C., Yeatman T., Balducci L., Ku N., Shivers S., Berman C., Wells K., Rapaport D. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996 Dec 11;276(22):1818–1822. [PubMed] [Google Scholar]
- Cady B. Is axillary lymph node dissection necessary in routine management of breast cancer? No. Important Adv Oncol. 1996:251–265. [PubMed] [Google Scholar]
- Copeland E. M., 3rd Is axillary dissection necessary for T1 carcinoma of the breast? J Am Coll Surg. 1997 Apr;184(4):397–398. [PubMed] [Google Scholar]
- Cox C. E., Ku N. N., Reintgen D. S., Greenberg H. M., Nicosia S. V., Wangensteen S. Touch preparation cytology of breast lumpectomy margins with histologic correlation. Arch Surg. 1991 Apr;126(4):490–493. doi: 10.1001/archsurg.1991.01410280094014. [DOI] [PubMed] [Google Scholar]
- Fisher B., Wolmark N., Bauer M., Redmond C., Gebhardt M. The accuracy of clinical nodal staging and of limited axillary dissection as a determinant of histologic nodal status in carcinoma of the breast. Surg Gynecol Obstet. 1981 Jun;152(6):765–772. [PubMed] [Google Scholar]
- Giuliano A. E., Dale P. S., Turner R. R., Morton D. L., Evans S. W., Krasne D. L. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995 Sep;222(3):394–401. doi: 10.1097/00000658-199509000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A. E., Jones R. C., Brennan M., Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997 Jun;15(6):2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- Giuliano A. E., Kirgan D. M., Guenther J. M., Morton D. L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994 Sep;220(3):391–401. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A. E. Lymphatic mapping and sentinel node biopsy in breast cancer. JAMA. 1997 Mar 12;277(10):791–792. [PubMed] [Google Scholar]
- Haffty B. G., Ward B., Pathare P., Salem R., McKhann C., Beinfield M., Fischer D., Reiss M. Reappraisal of the role of axillary lymph node dissection in the conservative treatment of breast cancer. J Clin Oncol. 1997 Feb;15(2):691–700. doi: 10.1200/JCO.1997.15.2.691. [DOI] [PubMed] [Google Scholar]
- Krag D. N., Weaver D. L., Alex J. C., Fairbank J. T. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993 Dec;2(6):335–340. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Aihara T., Motomura K., Inaji H., Imaoka S., Koyama H. Detection of breast cancer micrometastases in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Comparison between MUC1 mRNA and keratin 19 mRNA amplification. Am J Pathol. 1996 Feb;148(2):649–656. [PMC free article] [PubMed] [Google Scholar]
- Reintgen D. The credentialing of American surgery. Ann Surg Oncol. 1997 Mar;4(2):99–101. doi: 10.1007/BF02303789. [DOI] [PubMed] [Google Scholar]
- Ruffin W. K., Stacey-Clear A., Younger J., Hoover H. C., Jr Rationale for routine axillary dissection in carcinoma of the breast. J Am Coll Surg. 1995 Feb;180(2):245–251. [PubMed] [Google Scholar]
- Schoenfeld A., Luqmani Y., Smith D., O'Reilly S., Shousha S., Sinnett H. D., Coombes R. C. Detection of breast cancer micrometastases in axillary lymph nodes by using polymerase chain reaction. Cancer Res. 1994 Jun 1;54(11):2986–2990. [PubMed] [Google Scholar]
- Shabaik A. S., Cox C. E., Clark R. A., Reintgen D. S., Humphrey E. J., Nicosia S. V. Imprint cytology of needle-localized breast lesions. Acta Cytol. 1993 Jan-Feb;37(1):10–15. [PubMed] [Google Scholar]
- Turner R. R., Ollila D. W., Krasne D. L., Giuliano A. E. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997 Sep;226(3):271–278. doi: 10.1097/00000658-199709000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi U., Paganelli G., Galimberti V., Viale G., Zurrida S., Bedoni M., Costa A., de Cicco C., Geraghty J. G., Luini A. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997 Jun 28;349(9069):1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]