Abstract
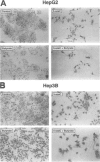
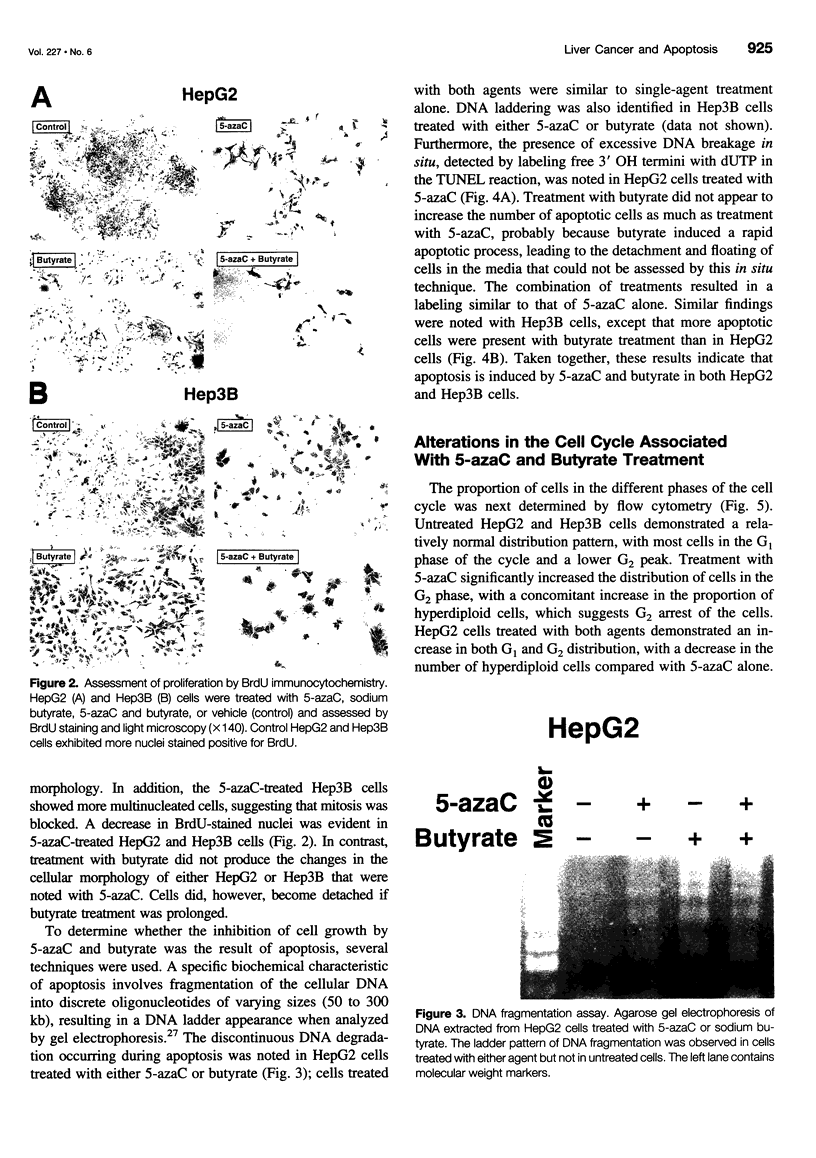
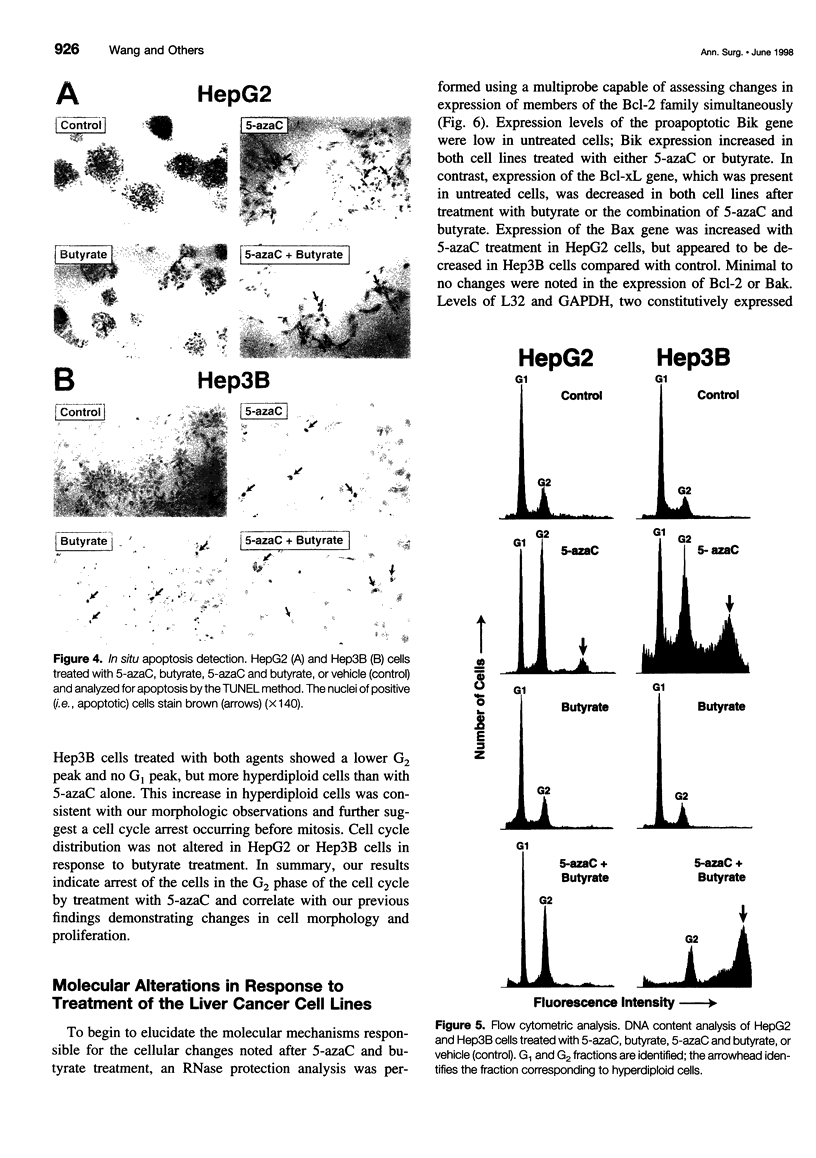
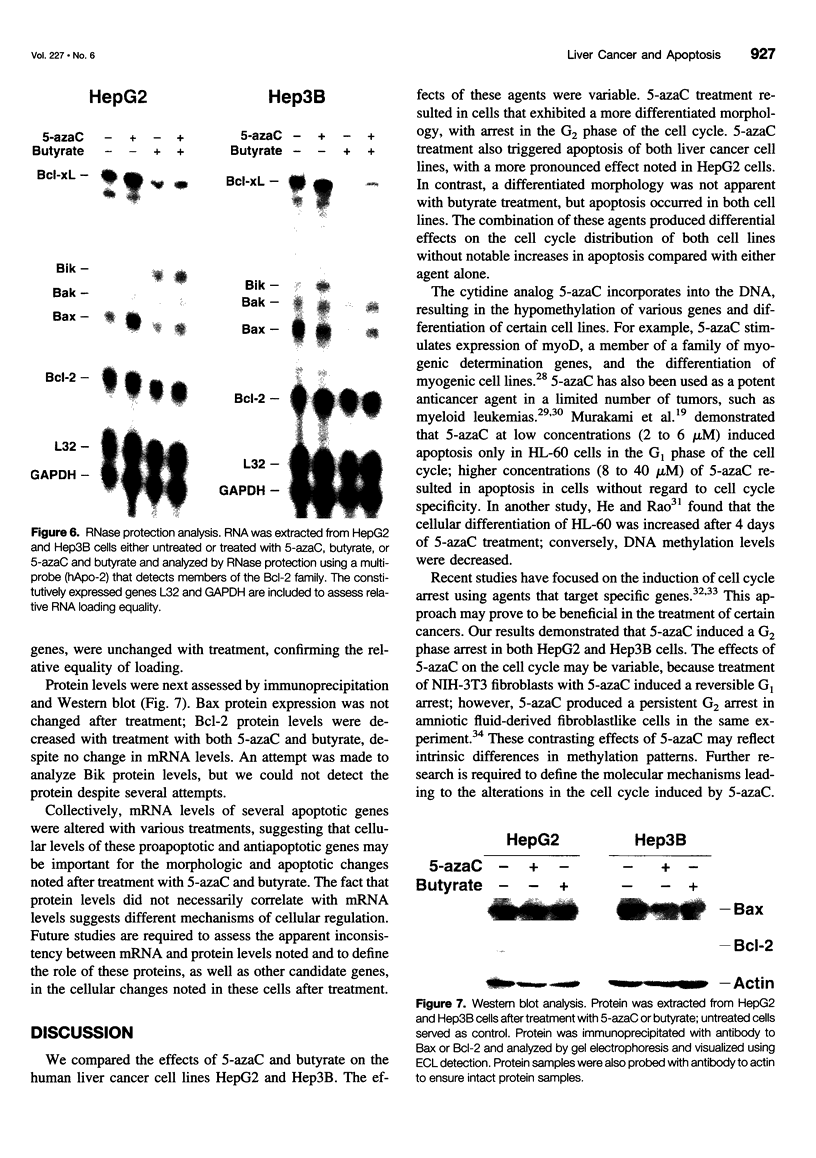
OBJECTIVE: To determine the cellular effects of 5-azacytidine (5-azaC) and sodium butyrate on two human liver cancers, HepG2 and Hep3B. SUMMARY BACKGROUND DATA: Primary liver cancer is a significant health problem; treatment options are limited and prognosis is poor. Recent studies have focused on the role that programmed cell death (i.e., apoptosis) plays in both normal and neoplastic growth: certain genes can either suppress (e.g., Bcl-2, Bcl-xL) or promote (e.g., Bik, Bax, Bak) apoptosis. The identification of novel agents targeted to specific molecular pathways may be beneficial in the treatment of this disease. METHODS: Human liver cancer cell lines HepG2 and Hep3B were treated with 5-azaC alone, butyrate alone, or 5-azaC and butyrate. Morphologic and proliferative changes were assessed by light microscopy and 5-bromo-2'-deoxyuridine staining; flow cytometry was used to determine cell cycle characteristics. Apoptosis was assessed by DNA laddering and the in situ apoptosis detection assay using the TdT-mediated dUTP nick end labeling method. In addition, total RNA and protein were analyzed by ribonuclease protection and Western blot, respectively, to assess changes in the expression of apoptosis-related genes. RESULTS: Treatment with either 5-azaC or butyrate inhibited cell growth and induced apoptosis in both HepG2 and Hep3B cells; the combination of 5-azaC and butyrate was not more effective than either agent alone. 5-azaC alone resulted in a more differentiated-appearing morphology and G2 cell cycle arrest in both cell lines. Treatment with 5-azaC or butyrate affected the expression levels of proteins of the Bcl-2 family. CONCLUSIONS: Both 5-azaC and butyrate induced apoptosis in the HepG2 and Hep3B liver cancer cells; 5-azaC treatment alone produced G2 arrest in both cell lines. Proteins of the Bcl-2 family may play a role in the cellular changes that occur with treatment, but further studies are required to define this potential role. Products of the apoptotic pathway may prove to be useful therapeutic targets in the treatment of hepatic cancers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Bellamy C. O., Malcomson R. D., Harrison D. J., Wyllie A. H. Cell death in health and disease: the biology and regulation of apoptosis. Semin Cancer Biol. 1995 Feb;6(1):3–16. doi: 10.1006/scbi.1995.0002. [DOI] [PubMed] [Google Scholar]
- Bhatia U., Traganos F., Darzynkiewicz Z. Induction of cell differentiation potentiates apoptosis triggered by prior exposure to DNA-damaging drugs. Cell Growth Differ. 1995 Aug;6(8):937–944. [PubMed] [Google Scholar]
- Boyd J. M., Gallo G. J., Elangovan B., Houghton A. B., Malstrom S., Avery B. J., Ebb R. G., Subramanian T., Chittenden T., Lutz R. J. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995 Nov 2;11(9):1921–1928. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cedar H., Razin A. DNA methylation and development. Biochim Biophys Acta. 1990 May 24;1049(1):1–8. doi: 10.1016/0167-4781(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Chang S. T., Yung B. Y. Potentiation of sodium butyrate-induced apoptosis by vanadate in human promyelocytic leukemia cell line HL-60. Biochem Biophys Res Commun. 1996 Apr 25;221(3):594–601. doi: 10.1006/bbrc.1996.0641. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conway R. M., Madigan M. C., Penfold P. L., Billson F. A. Induction of apoptosis by sodium butyrate in the human Y-79 retinoblastoma cell line. Oncol Res. 1995;7(6):289–297. [PubMed] [Google Scholar]
- Di Bisceglie A. M., Rustgi V. K., Hoofnagle J. H., Dusheiko G. M., Lotze M. T. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988 Mar;108(3):390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- Elangovan B., Chinnadurai G. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J Biol Chem. 1997 Sep 26;272(39):24494–24498. doi: 10.1074/jbc.272.39.24494. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Zhou Z., Celano P., Li J. The neurotensin gene is a downstream target for Ras activation. J Clin Invest. 1995 Jun;95(6):2822–2830. doi: 10.1172/JCI117987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E. p53-dependent repression of cdk4 synthesis in transforming growth factor-beta-induced G1 cell cycle arrest. J Lab Clin Med. 1996 Oct;128(4):355–360. doi: 10.1016/s0022-2143(96)80006-0. [DOI] [PubMed] [Google Scholar]
- Gupta S., Alpini G., Vemuru R. P., Hurston E., Shafritz D. A. Butyrate synchronization of hepatocytes: modulation of cycling and cell cycle regulated gene expression. Growth Factors. 1994;10(3):171–180. doi: 10.3109/08977199409000235. [DOI] [PubMed] [Google Scholar]
- Hague A., Elder D. J., Hicks D. J., Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer. 1995 Jan 27;60(3):400–406. doi: 10.1002/ijc.2910600322. [DOI] [PubMed] [Google Scholar]
- Han J., Sabbatini P., White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol. 1996 Oct;16(10):5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. J., Vaux D. L. Analysis of the role of bcl-2 in apoptosis. Immunol Rev. 1994 Dec;142:127–139. doi: 10.1111/j.1600-065x.1994.tb00886.x. [DOI] [PubMed] [Google Scholar]
- He Z. X., Rao H. The effect of 5-azacytidine (5-aza-CR) and its analogue on cell differentiation and DNA methylation of HL-60 cells. Sci China B. 1993 Apr;36(4):430–438. [PubMed] [Google Scholar]
- Heerdt B. G., Houston M. A., Augenlicht L. H. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ. 1997 May;8(5):523–532. [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Schaffner W. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 1989 May;3(5):612–619. doi: 10.1101/gad.3.5.612. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kritz A. D., Raptis G., Menendez-Botet C., Maslak P., Jakubowski A. Pilot study of 5-azacytidine (5-AZA) and carboplatin (CBDCA) in patients with relapsed/refractory leukemia. Am J Hematol. 1996 Feb;51(2):117–121. doi: 10.1002/(SICI)1096-8652(199602)51:2<117::AID-AJH4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Mandal M., Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell Growth Differ. 1996 Mar;7(3):311–318. [PubMed] [Google Scholar]
- Mandal M., Wu X., Kumar R. Bcl-2 deregulation leads to inhibition of sodium butyrate-induced apoptosis in human colorectal carcinoma cells. Carcinogenesis. 1997 Jan;18(1):229–232. doi: 10.1093/carcin/18.1.229. [DOI] [PubMed] [Google Scholar]
- McBain J. A., Eastman A., Nobel C. S., Mueller G. C. Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol. 1997 May 9;53(9):1357–1368. doi: 10.1016/s0006-2952(96)00904-5. [DOI] [PubMed] [Google Scholar]
- Murakami T., Li X., Gong J., Bhatia U., Traganos F., Darzynkiewicz Z. Induction of apoptosis by 5-azacytidine: drug concentration-dependent differences in cell cycle specificity. Cancer Res. 1995 Jul 15;55(14):3093–3098. [PubMed] [Google Scholar]
- Nerenstone S. R., Ihde D. C., Friedman M. A. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev. 1988 Mar;15(1):1–31. doi: 10.1016/0305-7372(88)90007-2. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1997. CA Cancer J Clin. 1997 Jan-Feb;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- Pinto A., Zagonel V. 5-Aza-2'-deoxycytidine (Decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: past, present and future trends. Leukemia. 1993 May;7 (Suppl 1):51–60. [PubMed] [Google Scholar]
- Reed J. C. Mechanisms of Bcl-2 family protein function and dysfunction in health and disease. Behring Inst Mitt. 1996 Oct;(97):72–100. [PubMed] [Google Scholar]
- Rivero J. A., Adunyah S. E. Sodium butyrate induces tyrosine phosphorylation and activation of MAP kinase (ERK-1) in human K562 cells. Biochem Biophys Res Commun. 1996 Jul 25;224(3):796–801. doi: 10.1006/bbrc.1996.1102. [DOI] [PubMed] [Google Scholar]
- Satterwhite D. J., Moses H. L. Mechanisms of transforming growth factor-beta 1-induced cell cycle arrest. Invasion Metastasis. 1994;14(1-6):309–318. [PubMed] [Google Scholar]
- Schroy P. C., Rustgi A. K., Ikonomu E., Liu X. P., Polito J., Andry C., O'Keane J. C. Growth and intestinal differentiation are independently regulated in HT29 colon cancer cells. J Cell Physiol. 1994 Oct;161(1):111–123. doi: 10.1002/jcp.1041610114. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Lamprecht S. A., Polak-Charcon S., Niv Y., Kim Y. S. Induction of the differentiated phenotype in human colon cancer cell is associated with the attenuation of subcellular tyrosine phosphorylation. Oncol Res. 1995;7(6):277–287. [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Sherman M. Hepatocellular carcinoma. Gastroenterologist. 1995 Mar;3(1):55–66. [PubMed] [Google Scholar]
- Taylor S. M. 5-Aza-2'-deoxycytidine: cell differentiation and DNA methylation. Leukemia. 1993 May;7 (Suppl 1):3–8. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Thomas G. L., Henley A., Rowland T. C., Sahai A., Griffin M., Birckbichler P. J. Enhanced apoptosis in transformed human lung fibroblasts after exposure to sodium butyrate. In Vitro Cell Dev Biol Anim. 1996 Sep;32(8):505–513. doi: 10.1007/BF02723054. [DOI] [PubMed] [Google Scholar]
- Vincent-Fiquet O., Rogez J. C., Boitte F., Brazier M., Desmet G. Effects of arginine butyrate and tributyrylxylitol on cultured human sarcoma cells. Anticancer Res. 1994 Sep-Oct;14(5A):1823–1828. [PubMed] [Google Scholar]
- Weller E. M., Poot M., Hoehn H. Induction of replicative senescence by 5-azacytidine: fundamental cell kinetic differences between human diploid fibroblasts and NIH-3T3 cells. Cell Prolif. 1993 Jan;26(1):45–54. doi: 10.1111/j.1365-2184.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Xiao H., Hasegawa T., Miyaishi O., Ohkusu K., Isobe K. i. Sodium butyrate induces NIH3T3 cells to senescence-like state and enhances promoter activity of p21WAF/CIP1 in p53-independent manner. Biochem Biophys Res Commun. 1997 Aug 18;237(2):457–460. doi: 10.1006/bbrc.1997.7158. [DOI] [PubMed] [Google Scholar]
- Zimra Y., Wasserman L., Maron L., Shaklai M., Nudelman A., Rephaeli A. Butyric acid and pivaloyloxymethyl butyrate, AN-9, a novel butyric acid derivative, induce apoptosis in HL-60 cells. J Cancer Res Clin Oncol. 1997;123(3):152–160. doi: 10.1007/BF01214667. [DOI] [PubMed] [Google Scholar]
- Zoref-Shani E., Lavie R., Bromberg Y., Beery E., Sidi Y., Sperling O., Nordenberg J. Effects of differentiation-inducing agents on purine nucleotide metabolism in an ovarian cancer cell line. J Cancer Res Clin Oncol. 1994;120(12):717–722. doi: 10.1007/BF01194269. [DOI] [PubMed] [Google Scholar]